Diagnostics: Page 6
-
Illumina CFO steps down amid executive shakeup
Ankur Dhingra, who worked with Illumina’s CEO at Agilent Technologies, is joining the genome sequencing company to replace Joydeep Goswami.
By Nick Paul Taylor • April 10, 2024 -
Exact Sciences’ cancer blood test achieves 51% sensitivity in prospective trial
Sensitivity for the multi-cancer early detection test rose to almost 64% in the six cancers with the shortest five-year survival rate.
By Nick Paul Taylor • April 9, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Physicians ask FDA to revoke approval of DNA test for opioid addiction
Physicians said the test is based “on old genetic studies that have largely been abandoned” and could exacerbate the opioid crisis.
By Nick Paul Taylor • April 8, 2024 -
Freenome’s colorectal cancer blood test data underwhelm analysts
Cowen analysts said Freenome’s results are good news for competitors Exact Sciences and Guardant Health.
By Nick Paul Taylor • April 5, 2024 -
Beckman Coulter receives FDA warning letter
Inspectors found fault with quality practices at a facility that makes immunoassay analyzer instruments and tests.
By Nick Paul Taylor • April 5, 2024 -
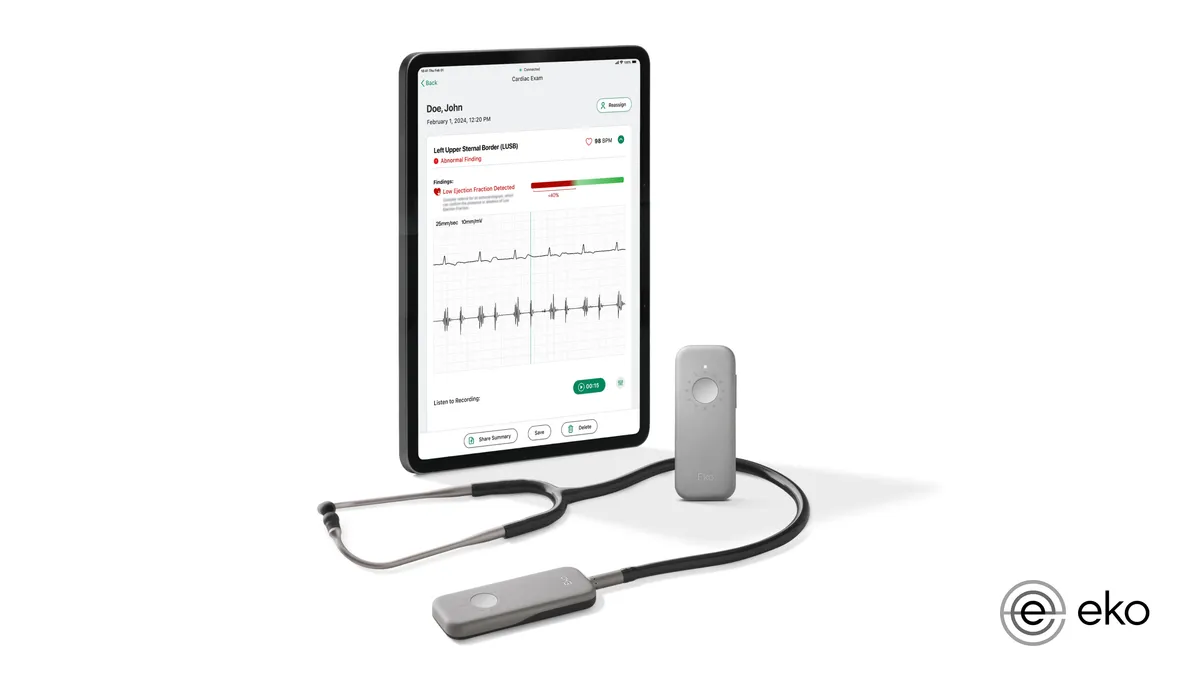
Eko wins FDA nod for AI to detect sign of heart failure using stethoscope
The clearance adds to the list of devices the FDA has authorized this year with AI algorithms to detect health conditions.
By Nick Paul Taylor • April 4, 2024 -
QuidelOrtho pulls FDA submission of 4-virus test
The diagnostics company said it is developing a next-generation combination respiratory assay after data for the initial test did not meet its expectations.
By Susan Kelly • April 3, 2024 -
Abbott wins FDA clearance for bedside blood concussion test
The clearance is a step towards using the test in non-healthcare settings, such as sporting events, Abbott said.
By Nick Paul Taylor • April 2, 2024 -
Exact Sciences, Mayo Clinic share early data on esophageal cancer test
Designed as an alternative to endoscopy, the cancer screening test uses a sponge collection device that's paired with an algorithm.
By Elise Reuter • March 29, 2024 -
Labcorp inks $237.5M buyout of Opko’s reproductive, women’s health testing assets
The acquired assets generate about $100 million in annual revenue, according to the deal announcement.
By Nick Paul Taylor • March 29, 2024 -
Hologic to lay off 190 workers as it closes European facilities
Three months after announcing unspecified international facility closures, Hologic has disclosed the number of affected employees and estimated $12.2 million in severance costs.
By Susan Kelly • Updated May 6, 2024 -
Roche wins FDA approval for first molecular malaria blood donor screening test
The company is pitching the test as a way to improve the safety and availability of blood.
By Nick Paul Taylor • March 28, 2024 -
Court adviser sides with Illumina in EU row that triggered Grail fine
Illumina is still planning to divest Grail but may escape a fine of about 432 million euros imposed by the European Commission.
By Nick Paul Taylor • March 25, 2024 -
As FDA’s LDT rule looms, experts warn patients may lose access to critical tests
In testimony to Congress Thursday, industry and patient group leaders described the trade-offs expected from increased FDA oversight of laboratory-developed tests.
By Susan Kelly • March 22, 2024 -
Thermo Fisher, Bayer partner to develop companion diagnostics
The companies aim to identify potential patients for Bayer’s precision cancer therapies and increase access to the treatments.
By Nick Paul Taylor • March 21, 2024 -
Siemens Healthineers to close fast track diagnostics unit, lay off about 90 workers
Demand for the products peaked during the COVID-19 pandemic but has fallen away since then.
By Nick Paul Taylor • March 19, 2024 -
Guardant posts data for colon cancer blood test amid push from rivals
The NEJM paper presents data from a study of more than 10,000 people Guardant ran to support authorization of its Shield DNA test.
By Nick Paul Taylor • March 18, 2024 -
QuidelOrtho to lay off employees after firing CEO
Interim CEO Michael Iskra said in a recent presentation to investors the diagnostics firm plans to lay off less than 10% of its staff.
By Susan Kelly • March 14, 2024 -
FDA panel backs Lumicell’s agent for breast cancer imaging tool
One panelist who voted yes said the “incremental benefits outweigh the small risks of anaphylaxis.”
By Nick Paul Taylor • March 11, 2024 -
Dexcom, Novo Nordisk call for FDA input on digital diabetes detection devices
The companies want clarity on what evidence would be needed for new technologies to detect undiagnosed Type 2 diabetes or prediabetes.
By Nick Paul Taylor • March 4, 2024 -
BD life sciences president Dave Hickey to retire July 1
The company has yet to name Hickey’s replacement but plans to have a successor in place before the executive retires.
By Nick Paul Taylor • Feb. 28, 2024 -
Natera secures Medicare coverage for cancer test in 2 new indications
Natera met the coverage requirements for ovarian cancer in adjuvant and surveillance settings and for breast cancer in the neoadjuvant setting.
By Nick Paul Taylor • Feb. 27, 2024 -
European Council approves extension of IVDR transition
The Council expects the European Parliament to vote on the plan, without seeking amendments, in April.
By Nick Paul Taylor • Feb. 26, 2024 -
QuidelOrtho fires CEO Doug Bryant
Bryant, who led the company since 2009, was fired after a challenging quarter and confusion over earnings forecasts.
By Elise Reuter • Feb. 21, 2024 -
UK medical device reviewers form new association
The group intends to help the medical device sector navigate a post-Brexit regulatory landscape.
By Susan Kelly • Feb. 20, 2024