Diagnostics: Page 23
-
BioCardia, Cook Medical land FDA breakthrough nods in latest designations
Cardiovascular devices feature prominently in the tech granted regulatory privileges, including a drug-eluting stent for patients with chronic limb-threatening ischemia and a non-invasive cardiac monitoring device.
By Nick Paul Taylor • Feb. 9, 2022 -
Q&A
Guardant Health targets colorectal cancer screening in 2022
CEO Helmy Eltoukhy tells MedTech Dive the precision oncology company plans to launch a liquid biopsy in the first half of the year, rivaling Exact Sciences' Cologuard stool test.
By Greg Slabodkin • Feb. 8, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
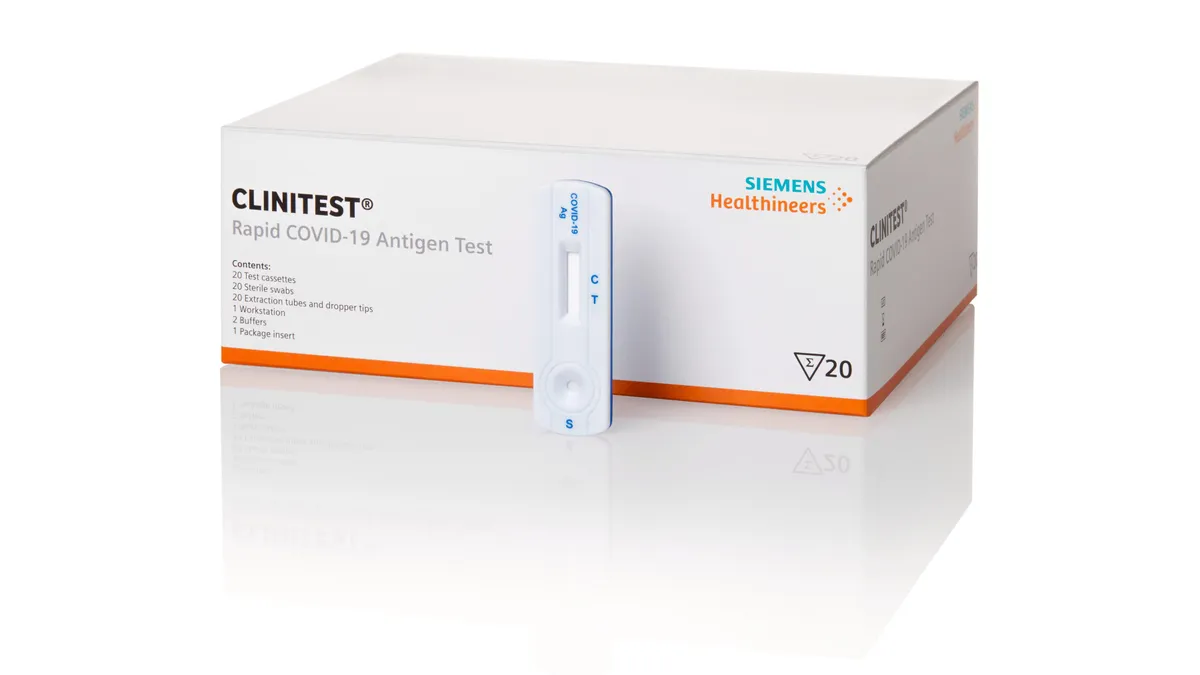
Roche partner recalls illegally imported rapid COVID-19 tests from US
FDA said there are "confirmed reports" of unlawful importation of the Standard Q COVID-19 Ag Home Test and South Korea's SD Biosensor is looking into which distributors or people were involved.
By Nick Paul Taylor • Feb. 7, 2022 -
CDRH targets hiring, novel approvals among 2022-2025 strategic priorities
FDA's Center for Devices and Radiological Health, whose staff continues to struggle with an unprecedented workload since the pandemic's start, is aiming to achieve at least 90% of its annual hiring targets for certain years.
By Nick Paul Taylor • Feb. 4, 2022 -
BD's Q1 revenue beats Street despite COVID-19 testing decline
In spite of the pandemic and macroeconomic challenges, CEO Tom Polen said the company has "confidence" to raise 2022 revenue guidance "while remaining appropriately prudent, given the current uncertain environment."
By Greg Slabodkin • Feb. 3, 2022 -
Thermo Fisher, Hologic plan for extended test demand in 2022
In a year of ups and downs for COVID-19 testing, both companies are raising revenue guidance for 2022 as the need for diagnostics isn't fading.
By Elise Reuter • Feb. 3, 2022 -
Siemens Healthineers ups 2022 forecast after COVID-19 test boom, but sees increased competition
CFO Jochen Schmitz told investors Thursday that as competition in the diagnostics space heats up this year, the company expects "revenues to decline sharply in the second half."
By Nick Paul Taylor • Feb. 3, 2022 -
Breakthrough device program 'far exceeding' FDA expectations after record year
The total number of products granted the agency's regulatory privileges in 2021 increased by more than 50%, while the number of novel devices that came to market fell compared to 2020.
By Nick Paul Taylor • Feb. 1, 2022 -
Deep Dive
3 key FDA topics for medtechs in 2022
While the agency is looking to get back to normal operations this year, COVID-19 is poised to remain a top priority and, once again, thin resources for more traditional work, such as product reviews.
By Ricky Zipp , Greg Slabodkin • Feb. 1, 2022 -
Roundup: Latest earnings show medtechs grappling with healthcare staffing shortages, supply chain
Medical device companies ended 2021 on an "uninspiring" note, J.P. Morgan analysts said, as they brace for a slowdown in procedures driven by a surge in COVID-19 cases due to the omicron variant.
By Elise Reuter • Jan. 31, 2022 -
iHealth Lab gets DoD order for additional 104M home COVID-19 tests
The transaction brings the total number of kits the Department of Defense has ordered from iHealth Lab to more than 350 million, putting the company at the heart of the Biden administration's effort to boost testing capacity.
By Nick Paul Taylor • Jan. 31, 2022 -
Abbott Q4 revenue beats estimates on demand for COVID-19 tests; company warns of 2022 uncertainty
Abbott provided an initial coronavirus test sales forecast of $2.5 billion, which is expected to occur early in the year and will be updated quarterly. Evercore ISI analysts said the company's "testing assumptions seem derisked."
By Greg Slabodkin • Jan. 26, 2022 -
FDA finalizes two guidances on including patient perspectives in medtech clinical trials
The documents, which elaborate on how to engage patients to improve trial design and use patient-reported outcomes, are based on feedback from groups like AdvaMed which raised concerns about "significant" legal issues.
By Nick Paul Taylor • Jan. 26, 2022 -
GE Healthcare's revenue hit by continued supply chain challenges
GE CEO Larry Culp said Tuesday that ongoing supply chain disruptions were "most acute" in healthcare and will last through at least the first half of 2022, describing the persistent problem as the worst in decades.
By Greg Slabodkin • Jan. 25, 2022 -
Medtech earnings season shows bumpy end to 2021 for some, bonanza for others
Procedure-dependent companies were negatively impacted to a greater or lesser degree by the surge in omicron cases in their most recent quarters, while COVID-19 test makers benefitted from the spike in demand.
By Elise Reuter • Jan. 24, 2022 -
Philips targets Q4 2022 end to recall as supply chain issues drag down results
The recall, which now impacts 5.2 million sleep apnea and ventilator machines, contributed to a 10% fall in comparable sales in the fourth quarter of 2021, while the increase in affected devices led Philips to up its field action provision.
By Nick Paul Taylor • Jan. 24, 2022 -
Diagnostics makers ride omicron surge in COVID-19 test demand. But for how long in 2022?
The highly transmissible variant caused the Biden administration to announce plans to buy 1 billion rapid tests to provide free to Americans. However, when testing will ramp back down remains an open question.
By Greg Slabodkin • Jan. 20, 2022 -
Exact Sciences posts positive data on Cologuard 2.0 ahead of readouts on rival tests
A study found Exact's second-generation stool test has a lower false positive rate than the current product and is better at detecting precancerous lesions. But it is facing competing blood tests from Freenome and Guardant Health.
By Nick Paul Taylor • Jan. 20, 2022 -
FDA misses MDUFA V deadline after months of contentious talks
The agency has failed to send the final MDUFA V user fee agreement to Congress by the Jan. 15 deadline, suggesting there may be unresolved disagreements with industry over elements of the program.
By Nick Paul Taylor • Jan. 19, 2022 -
Abbott, BD, Quidel pursue DTC strategies amid 'paradigm shift' for at-home, self-testing
The three test makers said at last week's J.P. Morgan healthcare conference that they see opportunities for direct-to-consumer, digitally-connected testing for other diseases beyond COVID-19.
By Greg Slabodkin • Jan. 18, 2022 -
Biden administration to buy 500M more rapid COVID-19 tests to give to Americans
The announcement by President Joe Biden now brings the administration's total purchase to 1 billion test kits. Abbott Laboratories, iHealth and Roche have so far been awarded contracts for a combined 380 million tests.
By Greg Slabodkin • Jan. 14, 2022 -
Medtech M&A, IPOs hit new highs as more buyers enter the space: SVB
In a new report, Silicon Valley Bank projects that medical device mergers and acquisitions "will stay hot, as a diverse set of acquirers compete for the top deals," but sees a slowdown in initial public offering activity.
By Nick Paul Taylor • Jan. 14, 2022 -
Neurological devices, diagnostics dominate latest FDA breakthrough designations
The agency has granted breakthrough designations to several devices for neurological conditions, including an autism test and treatment as well as diagnosis of Parkinson's disease.
By Nick Paul Taylor • Jan. 13, 2022 -
Notified body shortage remains pressing as industry prepares for staggered IVDR rollout
Three months have passed since the European Commission warned about a "grave shortage of notified body capacity," but the situation is largely unchanged.
By Nick Paul Taylor • Jan. 13, 2022 -
Biden health officials defend COVID-19 testing policies amid diagnostics shortage
Acting FDA Commissioner Janet Woodcock and others were under fire from senators during a Tuesday hearing for not doing enough to increase the availability of tests.
By Greg Slabodkin • Jan. 12, 2022