Diagnostics: Page 20
-
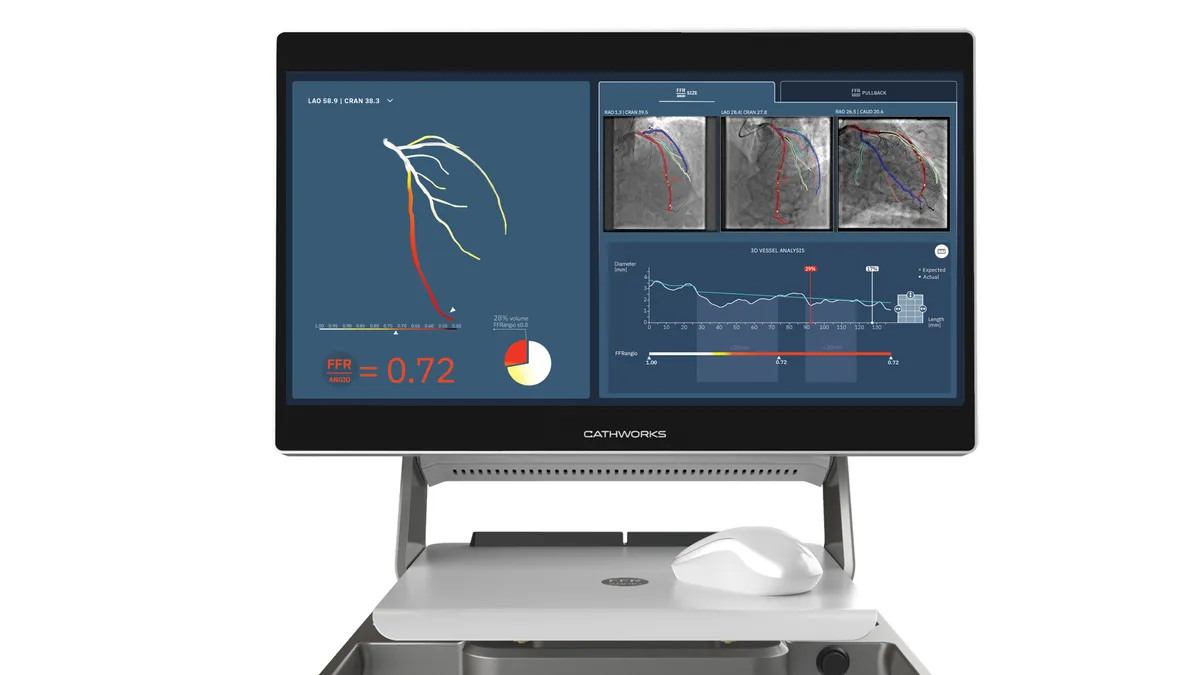
Medtronic lands option to buy artery disease player CathWorks for up to $585M
CathWorks has the right to compel Medtronic to complete the acquisition if it chooses not to exercise its option.
By Nick Paul Taylor • July 13, 2022 -
Hologic, Thermo Fisher roll out respiratory panels for Europe ahead of cold and flu season
Australia’s experience suggests Europe and other parts of the northern hemisphere may face high levels of respiratory disease this winter.
By Nick Paul Taylor • July 12, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
European Commission targets spring of 2024 for fully functional Eudamed database
The Commission is planning a two-phase transition to mandatory use of the database after it goes live.
By Nick Paul Taylor • July 11, 2022 -
Labcorp starts offering monkeypox testing using the CDC's PCR test
Labcorp claims it will become the first national laboratory to offer the test by beating its chief rival to market.
By Nick Paul Taylor • July 8, 2022 -
High-risk IVD tests rules finalized by European Commission despite industry calls for change
MedTech Europe pushed back against some of the requirements, but the Commission has retained disputed parts of the document.
By Nick Paul Taylor • July 8, 2022 -
SD Biosensor agrees to buy Meridian Bioscience for $1.53B in bid to enter U.S. IVD market
The Cincinnati-based diagnostics company says being acquired will help it enter new markets as part of SD Biosensor, as the COVID-detection business wanes.
By Elise Reuter • July 7, 2022 -
Semiconductor shortage leaves medtech industry 'more pessimistic' as customers leave, says Deloitte
Hospitals and health systems are looking into alternative products as a result of the disruption, according to a report from Deloitte and AdvaMed.
By Nick Paul Taylor • July 7, 2022 -
American Contract Systems' COVID-19 test recall gets Class I label from FDA
Off-site assembly by workers who may not have been properly trained prompted the company to recall COVID-19 tests amid concerns they may yield false results.
By Nick Paul Taylor • Updated July 6, 2022 -
COVID-19 antigen test sensitivity could be as low as 60% with omicron: FDA
The tests have taken a “really big hit in sensitivity” with the omicron variant, raising doubts about the ability of a single test to provide a definitive diagnosis, according to the agency.
By Elise Reuter • July 5, 2022 -
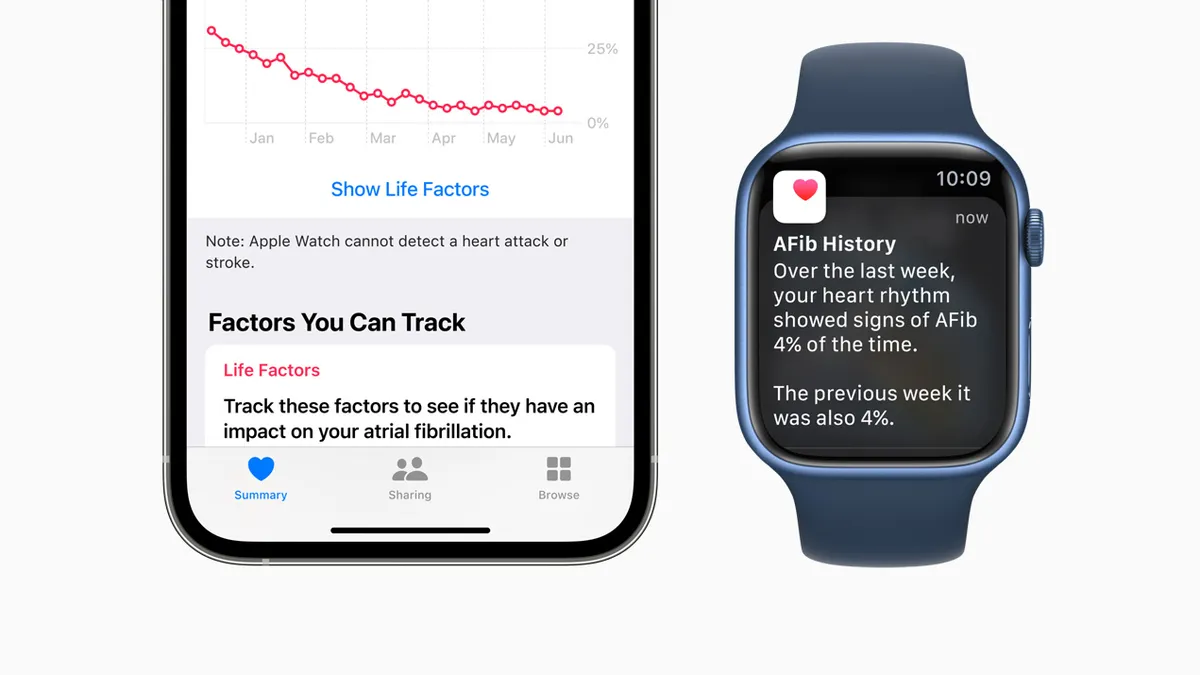
AliveCor ECG patent ruling sets stage for block on Apple Watch imports
Apple Watch imports to the U.S. could be barred if ruling by International Trade Court judge is finalized; Judge says Apple Watch infringes two cardiogram patents.
By Nick Paul Taylor • July 5, 2022 -
Q&A
Friday Q&A: Canary Medical CEO Bill Hunter discusses knee-implant sensors, device reimbursement
Canary’s founder discusses how the firm plans to create predictive tools from data collected by the devices, and long-term plans to embed its sensors in other implants.
By Elise Reuter • July 1, 2022 -

 Retrieved from LiveMetric website on July 01, 2022
Retrieved from LiveMetric website on July 01, 2022
LiveMetric's blood-pressure 'smartwatch' device gets FDA clearance
The wristwatch-like device will be made available through health systems, insurers and self-insured employers to ease continuous monitoring of blood pressure and avoid white-coat syndrome.
By Nick Paul Taylor • July 1, 2022 -
 Retrieved from Siemsens Website on July 01, 2022
Retrieved from Siemsens Website on July 01, 2022
Intuitive secures FDA clearance for lung biopsy robot featuring Siemens' imaging tech
The FDA clearance is an “incremental positive” for Intuitive, wrote analysts at RBC Capital Markets, adding that they see Ion as “an important leg of growth for the company.”
By Nick Paul Taylor • July 1, 2022 -
Commercial labs to ramp up monkeypox testing in 'coming weeks'
The Food and Drug Administration said in a Wednesday meeting that public health labs currently have a throughput of 10,000 tests per week, and the addition of five reference labs will expand that capacity to 60,000 tests per week.
By Elise Reuter • June 29, 2022 -
Cue Health cuts 170 jobs amid 'economic challenges,' decline in COVID-19 testing
With a contract to test the National Basketball Association, IPO funding and a $481 million government contract, Cue Health grew its headcount more than 10-fold in the pandemic.
By Nick Paul Taylor • June 28, 2022 -
UnitedHealth's Optum looks to cut down on unnecessary lab testing
Optum says roughly 13 billion clinical lab tests are performed each year and 30% are unnecessary.
By Samantha Liss • June 27, 2022 -
Sponsored by OptimizeRx
A digital point-of-care primer for medtech marketers
Technology advances are creating new opportunities in the care journey as digital point-of-care becomes part of patients’ and providers’ daily lives.
June 27, 2022 -
Medtech M&A falls 85% but activity could rebound in second half: report
PwC highlights a range of barriers that could stop acquisitions, including supply chain issues, scrutiny from the Federal Trade Commission and geopolitical concerns.
By Nick Paul Taylor • June 24, 2022 -
Lack of clinical evidence 'major gap' in digital health: study
The researchers framed the low scores as evidence of “a major gap in health care technology,” adding that there is a “significant opportunity” for companies that differentiate themselves with a more rigorous approach.
By Nick Paul Taylor • June 22, 2022 -
Senseonics lands CE mark for 6-month CGM implant, teeing up Q3 launch in Europe
Senseonics is looking to the longer-lasting implant to re-energize its fight in a market dominated by Abbott Laboratories and Dexcom.
By Nick Paul Taylor • June 17, 2022 -
User fee package goes to Senate with lab-developed test, OTC hearing aid provisions
The Senate HELP committee passed its version of the FDA user fee bill by a 13-9 vote. It includes an overhaul of diagnostic testing regulations and a requirement to create a category of over-the-counter hearing aids.
By Elise Reuter • June 15, 2022 -
DHS warns cybersecurity vulnerabilities in Illumina software could affect test results
Three of the flaws outlined by the Department of Homeland Security received the highest risk score. Vulnerabilities could allow attackers to remotely alter the results generated by Illumina products.
By Nick Paul Taylor • June 6, 2022 -
GE Healthcare says contrast media production back to full capacity
The shortage of the injectable used for medical imaging started in April, when the company’s plant in Shanghai shut down due to rising COVID-19 cases.
By Nick Paul Taylor • Updated June 17, 2022 -
FDA to start accepting all pre-submissions for in vitro diagnostics
The agency had previously declined pre-submission requests unless they were related to COVID-19. Test developers should expect an extended timeline for reviews amid a backlog of pandemic-related submissions.
By Elise Reuter • June 1, 2022 -
Baxter raises forecasts for Hillrom synergies, allaying concerns over $10.5B takeover
Company executives said the additional cost savings emerged as they took a closer look at Hillrom’s real estate footprint.
By Nick Paul Taylor • May 26, 2022