COVID-19: Page 36
-
EU MDR delay clears Parliament, enters final stages
During a plenary focused on combating the coronavirus pandemic, the European Parliament on Friday voted overwhelmingly in favor of pushing back the regulatory overhaul. A few steps remain.
By Nick Paul Taylor • April 17, 2020 -
GE, Ford get $336M HHS contract for 50K lower-cost ventilators
The latest deal works out to about $6,720 per ventilator. GE's roughly $64 million contract for 2,410 ventilators detailed earlier this week works out to a little more than $26,000 per device.
By Nick Paul Taylor • April 17, 2020 -
Abbott pulls 2020 guidance but expects coronavirus testing gains to show in Q2
"We haven't seen a quarter or any time quite like this before," new CEO Robert Ford told investors on an earnings call, during which he also addressed comments by some Trump administration officials on testing capacity.
By Greg Slabodkin • April 16, 2020 -
Boston Scientific to begin selling cheaper ventilator alternative
The "cereal box"-sized device OK'd by FDA will sell for a fraction of the price Philips is charging the U.S. government for its ventilators.
By Nick Paul Taylor • April 16, 2020 -

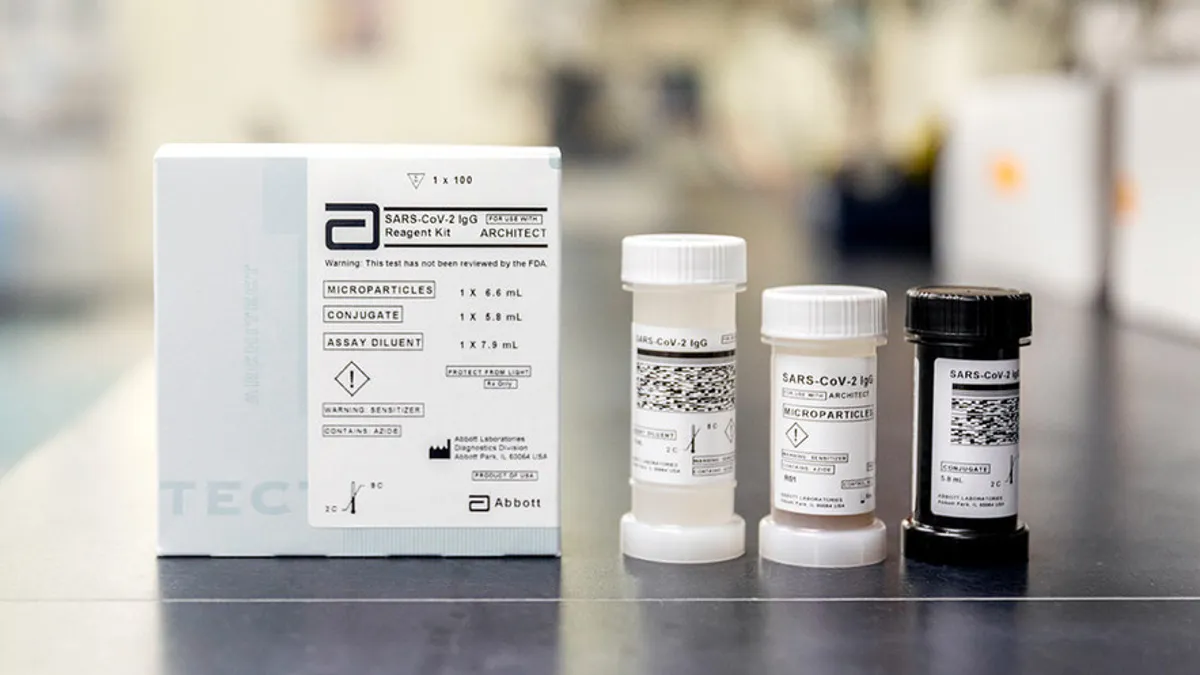
 Retrieved from Abbott, PRNewswire on March 19, 2020
Retrieved from Abbott, PRNewswire on March 19, 2020
CMS doubles Medicare payment for coronavirus lab tests
The American Clinical Lab Association praised the move to increase reimbursement for high-throughput molecular diagnostics, an effort to speed and expand COVID-19 testing nationwide.
By Greg Slabodkin • April 15, 2020 -
Device to wean COVID-19 patients off ventilators gains EUA
FDA said the Synapse Biomedical diaphragm stimulator could cut the time easing patients off the breathing support devices from four days to 2.6, with less time on a ventilator also cutting risk of secondary pneumonia.
By Susan Kelly • April 15, 2020 -
Abbott enters coronavirus antibody testing fray
The company will begin shipping tests tomorrow and plans to request an emergency use authorization from FDA, a status two new serology tests achieved Tuesday.
By Maria Rachal • April 15, 2020 -
Philips launches scaled-down ventilator, inks production deals to boost output
The medtech has allied with smaller players Flex and Jabil with a goal of 4,000 units per week by the third quarter and is also rolling out an alternative to its full-featured critical care breathing devices amid the coronavirus pandemic.
By Nick Paul Taylor • April 15, 2020 -
GE, Medtronic, ResMed among 7 medtechs part of $1.4B in finalized HHS ventilator contracts
Building on more than $1.1 billion announced for GM and Philips last week, the latest batch of contracts are aimed at producing over 64,000 of the potentially life-saving devices for COVID-19 patients.
By Maria Rachal • April 14, 2020 -
 CDC/Alissa Eckert, MS. "covid-19 coronavirus on white". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Alissa Eckert, MS. "covid-19 coronavirus on white". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
FDA authorizes 1st coronavirus test using at-home collection of saliva samples
The update to an EUA given last month for a test developed at Rutgers allows for at-home collection of specimens for the saliva-based test, although scope of the offering remains somewhat limited for now.
By Maria Rachal • Updated May 11, 2020 -
CMS removes ventilators from DME bidding program due to high coronavirus demand
Needham analysts said companies including ResMed, Hillrom and Inogen may benefit from the policy shift, given non-invasive ventilators could have faced reimbursement cuts as steep as 50% or more.
By Susan Kelly • April 14, 2020 -
J&J blames pandemic for medical device sales slump
CEO Alex Gorsky told investors Tuesday the company expects COVID-19 headwinds to continue "while elective procedures are deferred and hospital resources are redeployed to address patients impacted by this pandemic."
By Nick Paul Taylor • April 14, 2020 -
The latest coronavirus shortage: hospital infusion pumps
A surge in COVID-19 cases is not only straining the supply of the devices that administer medications and fluids, but the potentially risky tubing that allows them to be situated in hallways to create distance from sick patients' rooms.
By Greg Slabodkin • April 14, 2020 -
FDA EUAs go to 1st blood purifiers for COVID-19 patients, new N95 sterilizers
Terumo and CytoSorbents are behind blood purification devices designed to reduce inflammatory proteins in COVID-19 patients admitted to the ICU with confirmed or imminent respiratory failure.
By Susan Kelly • April 13, 2020 -

 "20-0081-031 (200318-N-BT681-1010)". Retrieved from Navy Medicine.
"20-0081-031 (200318-N-BT681-1010)". Retrieved from Navy Medicine.
CMS issues guidance to free patients from copays for coronavirus tests
Clinical labs worry Congress hasn't provided funds for the “free testing." But recent legislation gives the labs rates negotiated before the pandemic; or if there wasn't one, allows them to negotiate on price.
By Nick Paul Taylor • April 13, 2020 -
Medtechs adapt to new sales reality amid halt on elective surgeries, hospitals' financial stress
Device manufacturers can't control how long COVID-19 shuts down the procedures and traditional sales models they depend on. But their flexibility during the down period can make or break the restart, industry advisers say.
By Maria Rachal • April 9, 2020 -

 "White House Press Briefing". Retrieved from The White House.
"White House Press Briefing". Retrieved from The White House.
Over 90% of 1M Abbott coronavirus tests sitting idle, White House official says
Deborah Birx, coronavirus response coordinator, said Abbott's testing kits were only able to run 88,000 tests in three weeks because labs don’t have the resources to operate the company's high throughput systems at full capacity.
By Greg Slabodkin • April 9, 2020 -
EU permits remote notified body audits during pandemic
The guidance came the day after the Council of the EU responded to the European Commission's proposal for a one-year delay to the Medical Device Regulation, which Parliament is set to vote on next week.
By Nick Paul Taylor • April 9, 2020 -
Coronavirus chaos ripe for hackers to exploit medical device vulnerabilities
Interpol warned that cybercriminals are using ransomware to target healthcare organizations already overwhelmed by COVID-19, and noted a significant increase in detected health system attacks since the start of the pandemic.
By Greg Slabodkin • April 8, 2020 -
HHS, CVS, Walgreens get behind Abbott's coronavirus test
HHS has ordered 1,200 of Abbott's portable testing instruments and more than 30,000 of the point-of-care, quick-turnaround tests. The big retail pharmacy chains are also adopting the product as they expand drive-thru testing.
By Maria Rachal • April 8, 2020 -
 CDC/Hannah A Bullock; Azaibi Tamin. (2019). "covid-19 coronavirus microscopic image with blue colored viral particles". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Hannah A Bullock; Azaibi Tamin. (2019). "covid-19 coronavirus microscopic image with blue colored viral particles". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
OraSure wins BARDA contract for in-home coronavirus self-test
The test could become the first authorized by FDA for use at home to detect the virus, but its development won't likely be complete for at least another four to six months.
By Susan Kelly • April 8, 2020 -
Digital health rakes in record $3.1B in Q1, but coronavirus slump may loom
"Though some of the economic blow will be tempered by demand for connected healthcare, we do not anticipate investment activity to keep pace with Q1 levels in the coming months," a new Rock Health report says.
By Rebecca Pifer • April 7, 2020 -

 Retrieved from Flickr.
Retrieved from Flickr.
White House official urges caution before assuming coronavirus antibody test accuracy
The FDA last month made it easier for companies to quickly develop serology tests. But the Trump administration’s coordinator for coronavirus diagnostic testing warned against overreliance on the tests before they are validated.
By Greg Slabodkin • April 7, 2020 -
FDA OKs changes to oxygenation devices to treat coronavirus patients
A surge in patients with the sudden acute respiratory syndrome led to easing of allowed uses for cardiopulmonary bypass devices and ECMO machines. Medtronic and Getinge are among makers of the impacted devices.
By Nick Paul Taylor • April 7, 2020 -
FDA allows for infusion pump modifications to prevent shortages
In a bid to "reduce supply chain interruptions and manufacturing bottlenecks" during the pandemic, the agency will permit some changes to the pumps and accessories without the added step of a new 510(k) submission.
By Susan Kelly • April 6, 2020