COVID-19: Page 13
-
Talk of Baxter-Hillrom tie-up grows as both companies focus on connected care
Hillrom CEO John Groetelaars in a Friday earnings call highlighted the growing importance of connected care, the focus area of Baxter's current M&A strategy. A Baird analyst said it sounded like a non-specific defense of a deal.
By Nick Paul Taylor • Updated July 30, 2021 -
As Stryker boosted by electives comeback, CEO downplays delta variant risk to procedures
Kevin Lobo said the uncertainty is "baked in" to the company's forecast, though some analysts noted coronavirus variants cloud the outlook for the rest of the year.
By Susan Kelly • July 28, 2021 -
Thermo Fisher slashes COVID-19 testing guidance by $900M
Following peers like Abbott and Quidel, execs expect test demand to slow for the rest of the year. CFO Stephen Williamson called it "prudent" to "de-risk the outlook" for testing.
By Greg Slabodkin • July 28, 2021 -
GE Healthcare's COVID-19 recovery continues with double-digit growth in imaging, ultrasound
Second-quarter results reflect the return of elective procedures to pre-pandemic levels. A standout was its pharmaceutical diagnostics business which saw orders grow nearly 50% organically year over year.
By Greg Slabodkin • July 27, 2021 -
Boston Scientific tops pre-pandemic results in Q2, expects similar trends in H2
The company joins a handful of other medtechs seeming to move past the crisis, fueled by the return of elective care. CEO Michael Mahoney expects a "manageable level of COVID impact in the second half of the year."
By Ricky Zipp • July 27, 2021 -
Boston Scientific, Zimmer Q2 expectations lifted by Abbott, J&J, Intuitive results
Second-quarter results from Abbott Laboratories, J&J and Intuitive Surgical offer encouragement for the rest of the medtech reporting season, according to BofA Securities analysts. They also expect a "small beat" for Dexcom.
By Nick Paul Taylor • July 26, 2021 -
CDRH still digging out of backlog caused by COVID-19: Shuren
"We think it's more important to focus on the submissions we already have in house than to talk about products yet to come before us," the device center chief said. "Hopefully, sometime in 2022 we'll get back on track."
By Ricky Zipp • July 22, 2021 -
Abbott latest to report pandemic recovery in Q2 as procedures return, COVID-19 tests drag diagnostics
While growth was positive across the four major businesses, coronavirus test sales came in at $1.3 billion, far below the $2.2 billion reported in the previous quarter, though ahead of lowered expectations.
By Greg Slabodkin • July 22, 2021 -
FDA seeks funds, powers to fix 'great weaknesses' in medical device supply chain
Acting FDA Commissioner Janet Woodcock wants Congress to give the agency "expanded authority to obtain supply disruption notifications for critical devices any time there is the potential for a shortage."
By Nick Paul Taylor • July 22, 2021 -
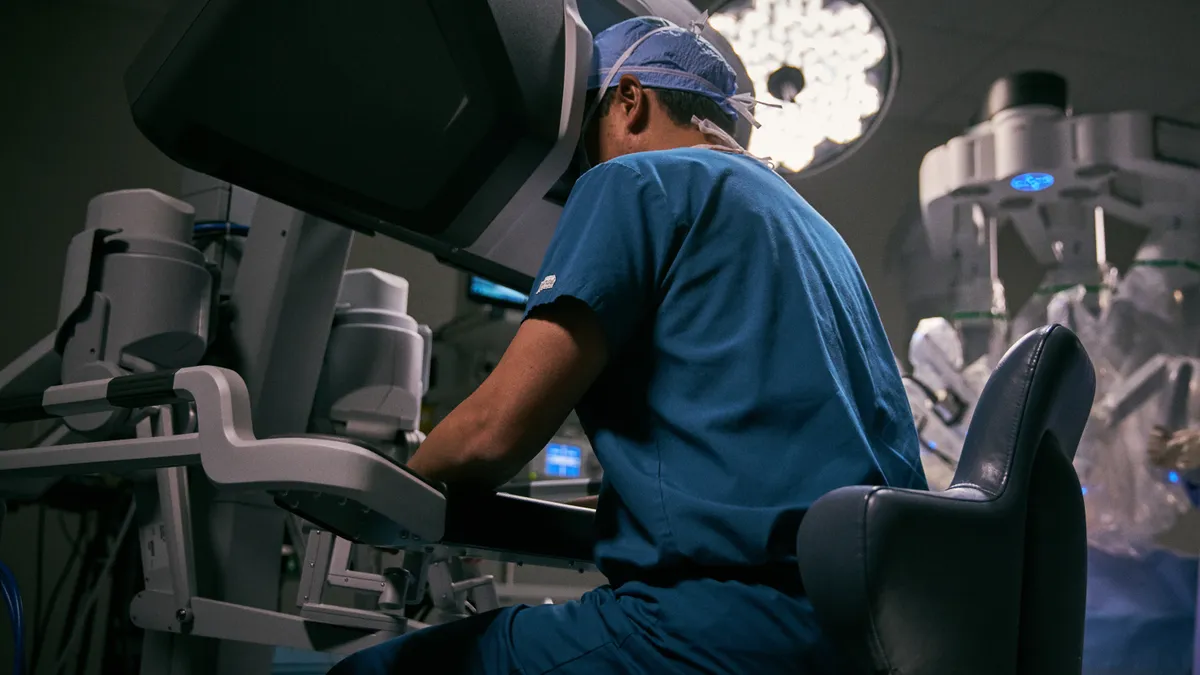
Intuitive's Q2 robot placements accelerate amid procedure recovery
While the robotic surgery leader is eyeing the delta variant for potential to curb demand, the company raised its forecast for procedure growth this year. However, the outlook does not reflect significant disruptions.
By Susan Kelly • July 21, 2021 -
J&J medical device sales return to pre-pandemic levels in Q2 as COVID-19 impact wanes
The company's return to growth, fueled by the recovery of elective volumes, could signal where procedure-dependent medtechs stand as the industry recovers from the effects of the coronavirus.
By Ricky Zipp • July 21, 2021 -
Medtech funding surges with early-stage deals
Robot company CMR Surgical, portable dialysis system maker Quanta Dialysis Technologies and diabetes technology developers were among the biggest recipients of private financing in the second quarter.
By Susan Kelly • July 20, 2021 -
Abbott, Intuitive and J&J kick off medtech earnings as COVID-19 bellwethers
Intuitive and J&J are set to report results this week that will shed light on the recovery of elective procedures, while Abbott will provide insights into the drop in coronavirus test demand.
By Nick Paul Taylor • July 19, 2021 -
Deep Dive
Medtech M&A expected to be robust in second half after 2021 began with a flurry
Even after deals already eclipsed last year's numbers, analysts predict robust activity in particular among diagnostics companies flush with cash from 2020's COVID-19 test sales.
By Ricky Zipp • July 18, 2021 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
FDA grants Curative request to revoke COVID-19 test EUA, company pivots to Abbott kits after false result risk
The agency, which announced the revocation on Thursday, originally granted an emergency use authorization to Curative's test in April 2020. By January, FDA had concerns about false results potentially linked to sample collection.
By Nick Paul Taylor • July 16, 2021 -
CMS proposes extension of Medicare telehealth coverage
Provider groups are not happy with the payment adjustment in the rule — a 3.75% reduction to the conversion factor due to budget neutrality requirements — and will likely seek congressional intervention.
By Shannon Muchmore • July 14, 2021 -
Telehealth use stabilizing at 38 times pre-COVID-19 levels, McKinsey says
Although usage has dropped slightly since its peak in spring 2020, patient and physician attitudes toward telehealth have improved, according to new data from the consultancy.
By Rebecca Pifer • July 13, 2021 -
Qiagen joins Abbott, Quidel cutting 2021 outlook as COVID-19 test demand wanes
CEO Thierry Bernard blamed a faster-than-expected testing drop and uptick of vaccinations. Jefferies said the downgraded targets are disappointing, particularly given management's forecast reiteration last month.
By Greg Slabodkin • July 13, 2021 -
Deep Dive
Medtech pay to doctors plunged in 2020 as COVID-19 pummeled electives, in-person services fell
Zimmer Biomet had the biggest drop, cutting general payments nearly 80% to $63 million. Other notable decreases include Stryker, J&J's DePuy Synthes and Boston Scientific.
By Ricky Zipp • July 12, 2021 -
Abbott lays off 400 workers amid drop in demand for COVID-19 tests
The company cut its 2021 outlook in June, blaming a sharp, rapid decline in demand for its coronavirus-related diagnostics on global vaccination efforts. The downward trend is anticipated to continue, an Abbott spokesperson said.
By Greg Slabodkin • July 9, 2021 -
Quidel COVID-19 PCR recall dubbed Class I by FDA due to false negative risk
The diagnostic company said it worked with FDA to confirm "a rare potential" for false test results and that the notice alerts lab customers of label changes made two months ago with no products removed from shelves.
By Nick Paul Taylor • Updated July 9, 2021 -
Q&A
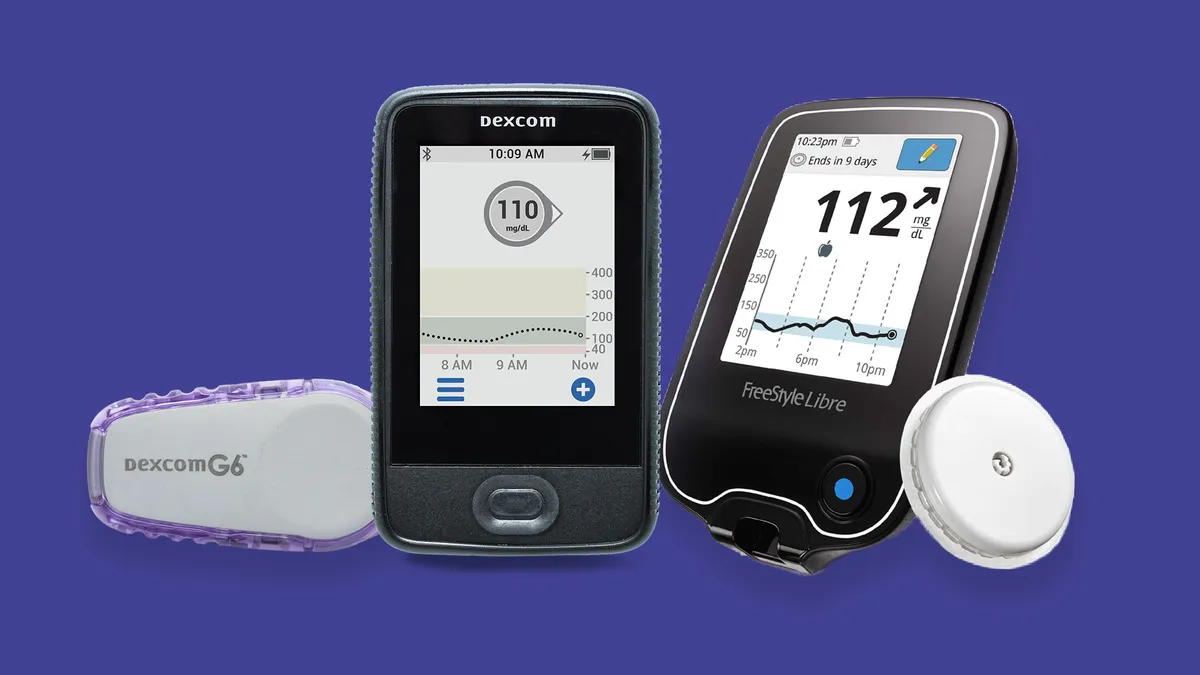
Dexcom CEO on the Type 2 population, the Super Bowl ad and pandemic momentum
Kevin Sayer called Type 2 a "tremendous market opportunity" and said direct-to-consumer advertising is worth some controversy.
By Ricky Zipp • July 6, 2021 -
PMA apps could hit record in 2021, pressuring a pandemic-stressed FDA
Despite increased workloads from the coronavirus crisis, the agency is also on track to issue record numbers of safety warnings and letters to healthcare providers in 2021, according to a Wells Fargo analysis.
By Nick Paul Taylor • July 2, 2021 -
COVID-19 antigen testing on par with PCR when used often: NIH-funded study
The two diagnostic methods were equally effective in detecting SARS-CoV-2 infection. The results could be good news for antigen test makers Abbott, Becton Dickinson and Quidel.
By Greg Slabodkin • July 1, 2021 -
Roundup: Trial results, looming product releases fuel diabetes tech competition
In the first half of the year, the space is meeting Wall Street's high expectations for 2021. Here's a roundup of MedTech Dive's coverage of the market, including from this week's American Diabetes Association meeting.
June 30, 2021