Clinical Trials: Page 6
-
Pivotal data on Guardant’s colorectal cancer blood test raise doubts about commercial prospects
Guardant’s share price fell 27% after the release of the news, which raised questions about whether the test can drive sales growth.
By Nick Paul Taylor • Dec. 19, 2022 -
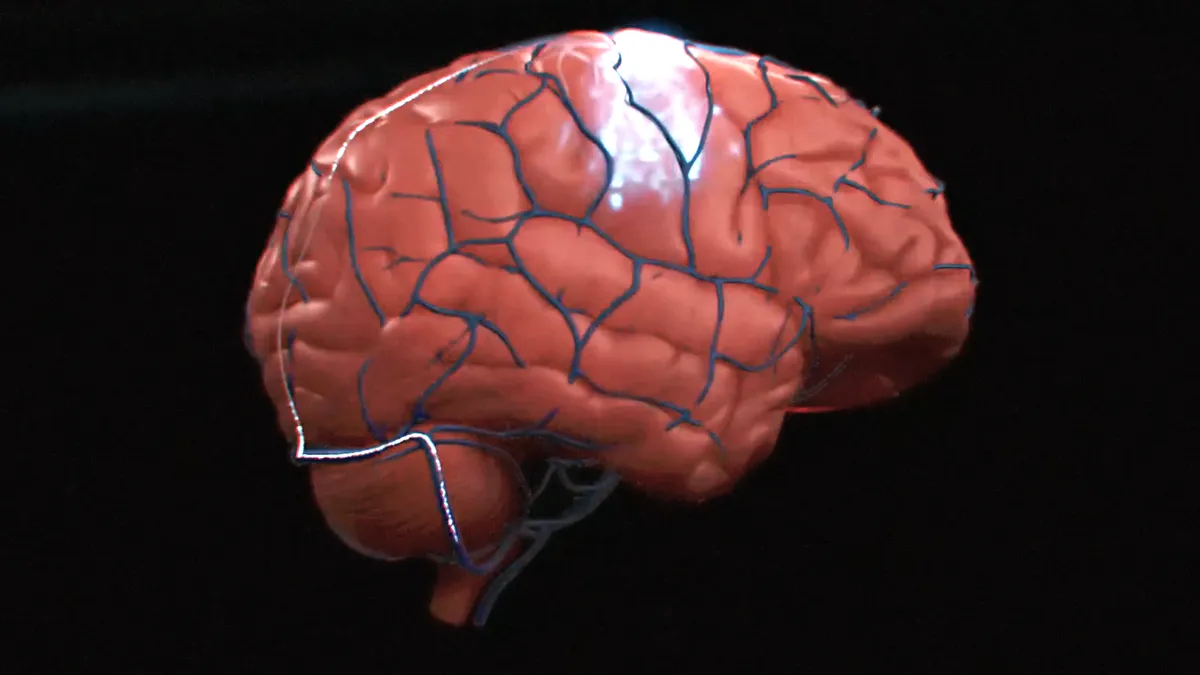
Gates, Bezos invest in Synchron’s brain computer interface
A $75 million investment will accelerate the development of a drill-free implant to help patients with upper-limb paralysis.
By Peter Green • Dec. 16, 2022 -
Investors hand Vaxxas $23M to support trial of needle-free COVID-19 vaccine
With support from the Gates Foundation, the needle-free technology could get more people vaccinated and avoid cold-chain issues.
By Nick Paul Taylor • Dec. 12, 2022 -
The 10 biggest medtech stories of 2022
MedTech Dive reporting this year has explored how companies maintained momentum even amid supply shortages and rising inflation rates.
By MedTech Dive staff • Dec. 3, 2022 -
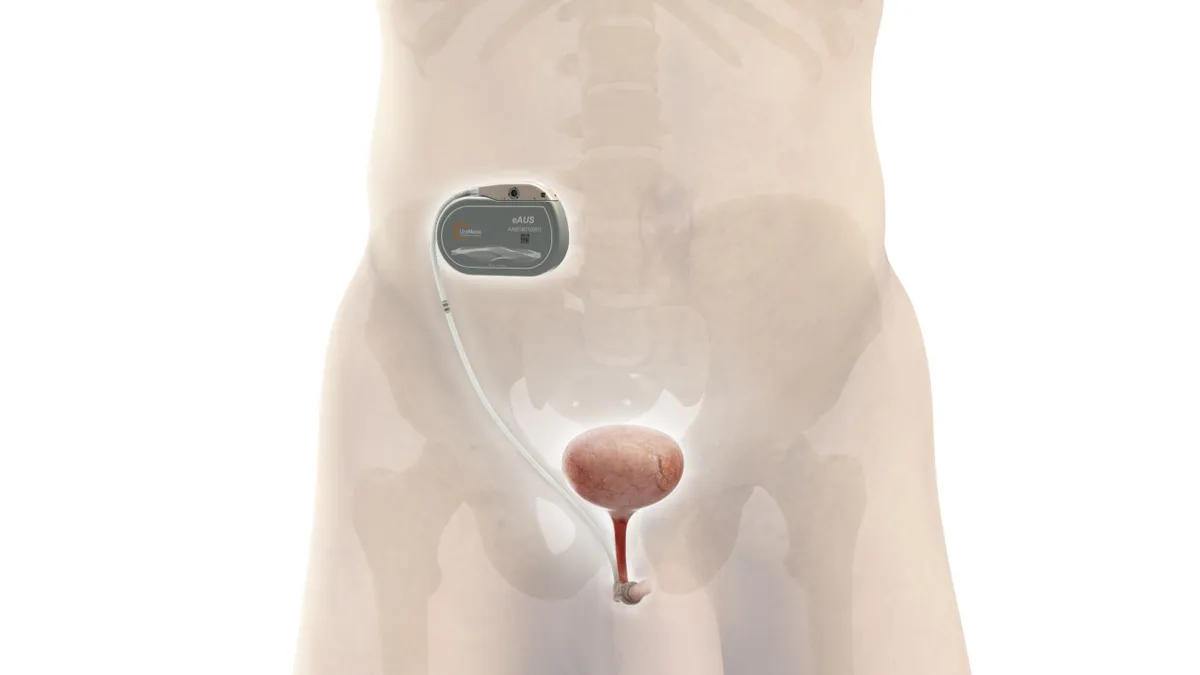
UroMems starts clinical trial of smart, automated implant to treat incontinence
The device is designed to improve on products such as Boston Scientific’s AMS 800 by eliminating the need for manual adjustments.
By Nick Paul Taylor • Nov. 30, 2022 -
Apple earbuds show promise as hearing aids in clinical trial
The earbuds performed comparably to hearing aids in terms of speech perception in quiet environments.
By Nick Paul Taylor • Nov. 22, 2022 -
Medtronic’s renal denervation treatment ‘still a viable therapy’ despite study miss, doctors say
While the study missed its primary endpoint, the surgery likely will be an option for patients with drug-resistant hypertension.
By Elise Reuter • Nov. 17, 2022 -
Tandem pump, paired with Dexcom CGM, boosts blood glucose control in Type 2 diabetics
Patients with Type 2 diabetes spent 3.6 hours per day longer in the target blood glucose range after switching to Tandem’s system, a study found.
By Nick Paul Taylor • Nov. 14, 2022 -
Green light shows promise in addressing oximetry racial bias: study
Researchers created a noninvasive device that performed comparably to Abbott’s invasive i-STAT in a small clinical trial.
By Nick Paul Taylor • Nov. 14, 2022 -
Affordable alternative: Smartphone-based hearing test for infants targets developing countries
The off-the-shelf alternative has a material cost of $10, giving children in impoverished regions easier access to vital monitoring and diagnosis.
By Nick Paul Taylor • Nov. 9, 2022 -
Medtronic misses primary endpoint in renal denervation study
The device didn't outperform drug therapy when patients measured their blood pressure at home, though it's still expected to win FDA approval.
By Elise Reuter • Nov. 8, 2022 -
Medtronic’s AFib ablation device beats antiarrhythmic drugs in 3-year study
Arctic Front cryoablation devices show promise as an early treatment for atrial fibrillation.
By Nick Paul Taylor • Nov. 8, 2022 -
Abiomed wraps up Impella post-approval studies requested by FDA
Abiomed’s studies linked the device to a 22% to 45% improvement in the volume of blood pumped after 90 days, suggesting it’s a viable alternative to balloon therapy.
By Nick Paul Taylor • Oct. 21, 2022 -
AI matches humans at detecting mental health red flags in text messages, study shows
The study suggests algorithms have the potential to enable automated tools for clinical support of people with mental illness.
By Nick Paul Taylor • Oct. 13, 2022 -
‘Do-it-yourself’ artificial pancreas system beats production-line pump in controlled trial
Type 1 diabetes patients who used a customizable, open-source artificial pancreas, an insulin pump and a Dexcom G6 CGM found it more effective at controlling blood glucose than conventional sensor-augmented insulin pumps.
By Nick Paul Taylor • Oct. 12, 2022 -
FDA finalizes postapproval study guidance in light of AdvaMed, Foundation Medicine feedback
The agency resisted calls to give sponsors more time to prepare protocols for post-approval studies.
By Nick Paul Taylor • Oct. 11, 2022 -
 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
Abbott’s FreeStyle Libre 2 CGM beats fingerstick testing in independent clinical trial
Going into the study, the researchers were unsure if CGMs with optional alarms for high and low blood glucose levels benefit patients with Type 1 diabetes.
By Nick Paul Taylor • Oct. 11, 2022 -
‘Inadequate’ progress of Bayer’s study on birth-control implant called out by FDA as patients drop out
The FDA saw a rise in patients dropping out of a study on the Essure birth-control implant, prompting it to tell Bayer to develop and implement strategies to ensure the work continues.
By Nick Paul Taylor • Oct. 7, 2022 -
Genentech, Winterlight track changes in Alzheimer’s using automated speech-based system
The researchers called the system a “promising prototype,” while cautioning that further validation is needed.
By Nick Paul Taylor • Oct. 5, 2022 -
Medical machine-learning studies lack high-quality clinical trials, review shows
The findings “highlight areas of concern regarding the quality of medical machine learning RCTs and suggest opportunities to improve reporting.”
By Nick Paul Taylor • Oct. 4, 2022 -

 National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
In surprise result, Alzheimer’s drug from Eisai, Biogen shows benefit in large trial
The companies said the drug, called lecanemab, met all of its goals in the Phase 3 study. The data are a significant finding and provide stronger support for a much-debated hypothesis for treating Alzheimer’s.
By Jonathan Gardner • Updated Sept. 27, 2022 -
Forget oximeters, smartphone cameras detect 79% of cases of low blood oxygen in small study
If the results are validated by wider studies, physicians will have a new tool to increase the number of people who can be monitored for chronic and acute respiratory conditions.
By Nick Paul Taylor • Sept. 23, 2022 -
Q&A
Friday Q&A: Scripps’ Jay Pandit talks about wearables, decentralized trials and what’s next for digital health
As wearables confirm their ability to collect data, the big question is what to do with it all. And the next technical challenge: improving wrist-based blood pressure monitors.
By Elise Reuter • Sept. 22, 2022 -
Abbott’s latest Amulet-Watchman data show more mixed results for the heart devices
The latest head-to-head trial adds to the growing rivalry between Abbott and Boston Scientific in the left atrial appendage closure space.
By Ricky Zipp • Sept. 19, 2022 -
Amid merger turmoil, Illumina’s Grail unit reports downturn in accuracy of blood-based cancer test
New data on Grail’s cancer screening test could weaken the company’s competitive standing against rivals Guardant Health, Exact Sciences and Freenome.
By Nick Paul Taylor • Sept. 12, 2022