Clinical Trials
-
Boston Scientific’s Farapulse matches Medtronic cryoablation in trial
Atrial tachyarrhythmia recurred in 39 patients treated with Boston Scientific’s Farapulse PFA device, while 53 people had recurrence in the Medtronic cryoablation group.
By Nick Paul Taylor • April 3, 2025 -
3 takeaways from ACC 2025
Medtronic, Abbott and Boston Scientific had updates on their devices used in heart valve disease treatment at the event.
By Susan Kelly • April 2, 2025 -
Abbott snags early CE mark for Volt PFA device
With PFA becoming physicians’ preferred ablation method for treating AFib, Abbott is pushing to catch up with rival systems already on the market.
By Susan Kelly • March 28, 2025 -
Academic medical centers say funding cuts jeopardize health research
Proposed cuts to National Institutes of Health funding threaten a critical pipeline of innovation and discovery necessary to fuel advancements in patient care, according to researchers and provider groups.
By Susanna Vogel • March 25, 2025 -
Abbott gets FDA nod to begin IVL trial after rivals buy up competition
J&J acquired the IVL device maker Shockwave Medical last year for $13.1 billion, and Boston Scientific agreed to buy Bolt Medical in January for up to $664 million.
By Nick Paul Taylor • March 25, 2025 -

 Retrieved from Spectral AI on March 18, 2025
Retrieved from Spectral AI on March 18, 2025
Spectral AI algorithms beat physicians in burn tissue assessments
The company plans to file the results with the FDA by mid-2025 and launch the product in 2026.
By Nick Paul Taylor • March 18, 2025 -
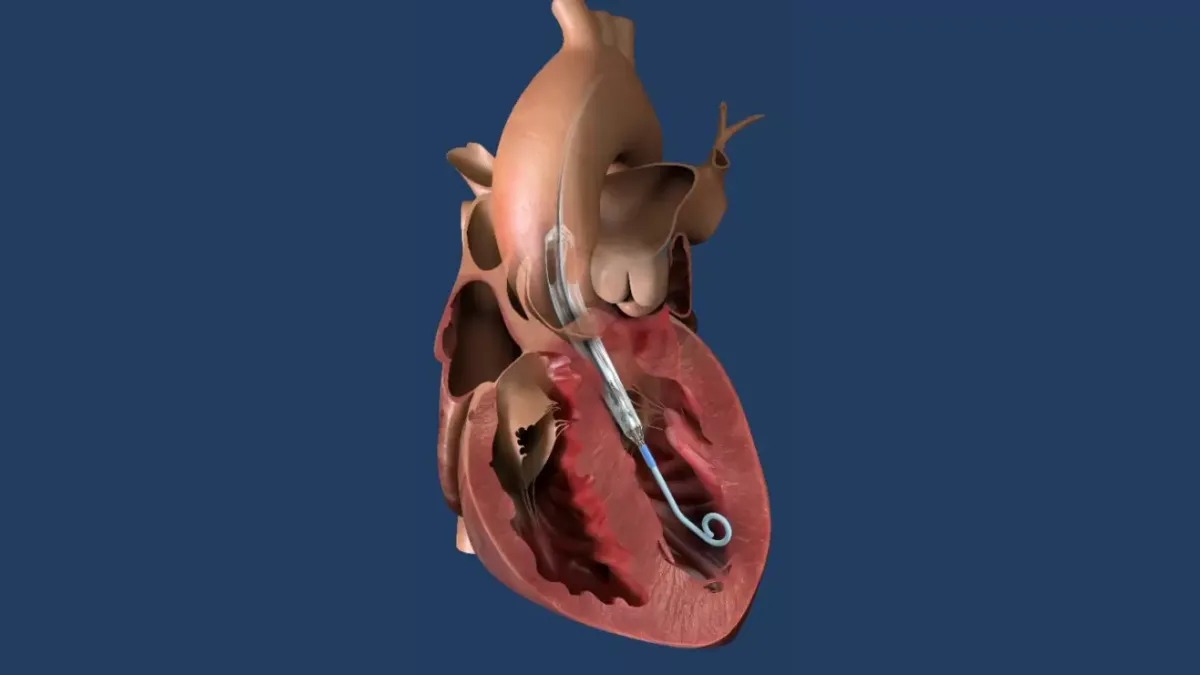
Capstan brings surgical robot to valve replacement
Capstan Medical, a startup focused on robotic-assisted mitral and tricuspid valve replacement, recently completed its first patient implants.
By Susan Kelly • March 11, 2025 -
EMA creates advice portal for some high-risk medical devices
The European Medicines Agency said the portal will enable consultations with medical device expert panels that can promote faster access to new technologies.
By Nick Paul Taylor • Feb. 12, 2025 -

 Retrieved from Screenshot: The Food and Drug Administration.
Retrieved from Screenshot: The Food and Drug Administration.
FDA webpages on clinical trial diversity removed after Trump orders
The website changes raise concerns about “the interference of politics with the study and the practice” of science and medicine, a physician said.
By Elise Reuter • Jan. 27, 2025 -
Medtronic links closed-loop pain device to improvements after 12 months
The Inceptiv data give Medtronic more evidence as it tries to increase sales of a product that drove double-digit growth at the company’s pain stimulation unit last quarter.
By Nick Paul Taylor • Jan. 24, 2025 -
‘Research that’s desperately needed’: White House conference spotlights women’s health
“Sometimes the most under-hyped sectors are the best places to build very large businesses,” said one venture investor who attended the Dec. 11 event.
By Delilah Alvarado • Dec. 19, 2024 -
Movano receives FDA nod for smart ring’s pulse oximeter feature
Movano Health plans to market the Evie Ring to organizations that run clinical trials and healthcare companies that are helping patients manage chronic diseases.
By Nick Paul Taylor • Dec. 3, 2024 -
Medtronic, Tempus testing AI to find potential TAVR patients
The randomized trial will use Tempus’ platform to find patients who are eligible for interventions such as TAVR but do not have a treatment plan in place.
By Nick Paul Taylor • Nov. 25, 2024 -
Boston Scientific’s Watchman could be new option for patients post ablation: study
The trial compared the stroke prevention device for left atrial appendage closure to blood thinners in people who underwent cardiac ablation for AFib.
By Susan Kelly • Nov. 18, 2024 -


Photo by MART PRODUCTION from Pexels

Livanova hits sleep apnea trial goals, plans FDA filing
Leerink analysts said the results were largely in line with outcomes in a trial of Inspire Medical’s rival device but cautioned that Livanova may have a hard time breaking into the sleep apnea market.
By Nick Paul Taylor • Nov. 13, 2024 -
J&J’s Ottava surgical robot to begin clinical trial after FDA nod
With FDA approval of an investigational device exemption, J&J’s soft tissue robot moves a step closer to challenging Intuitive Surgical’s da Vinci system.
By Susan Kelly • Nov. 12, 2024 -
Boston Scientific resumes PFA trial in new patient group after pausing enrollment
Boston Scientific expects to complete enrollment in the coming months in the study comparing pulsed field ablation to anti-arrhythmic drugs in patients with persistent AFib, a spokesperson said.
By Susan Kelly • Nov. 12, 2024 -
Edwards, Medtronic and Inari share trial data at TCT conference
The three medtech firms unveiled new data this week during TCT 2024 in Washington, D.C. Here’s what the results mean for the companies and their device markets.
By Nick Paul Taylor • Oct. 31, 2024 -
Boston Scientific’s Acurate Neo2 inferior to rival TAVR valves in study
Analysts said the findings complicate the outlook for a U.S. launch of Boston Scientific's aortic valve replacement device.
By Susan Kelly • Oct. 31, 2024 -
Edwards data show benefit of early TAVR over ‘watchful waiting’
Study author Philippe Genereux said the trial data “shatter 60 years of ingrained belief on the treatment for severe aortic stenosis.”
By Susan Kelly • Oct. 29, 2024 -
J&J to seek FDA approval after small-bore Impella heart pump hits trial goal
The company has predicted the narrower Impella ECP will be easier to insert and implant, as well as enable the use of small bore access and closure techniques.
By Nick Paul Taylor • Oct. 29, 2024 -
The Medtech Conference
That’s a wrap: 5 takeaways from The Medtech Conference
The conference — Advamed’s largest — featured an appearance by the device center’s new acting director and sessions on AI, clinical trial diversity and the FDA’s contentious LDT rule.
By Elise Reuter • Oct. 22, 2024 -
Abbott enrolls pivotal PFA study 4 months ahead of schedule
The company reported the enrollment milestone alongside news that it received clearance to sell its Advisor HD Grid X Mapping Catheter and started a trial of its Tactiflex Duo Ablation Catheter.
By Nick Paul Taylor • Oct. 11, 2024 -
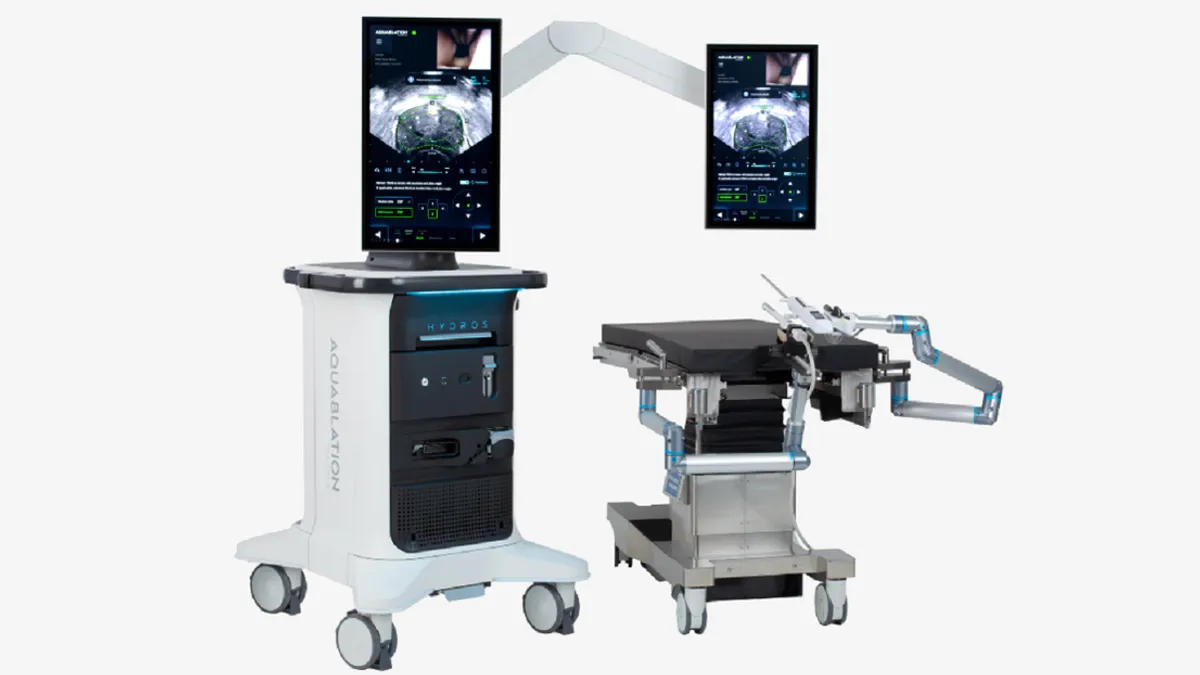
Procept secures FDA approval to study surgical robot in prostate cancer
The trial will compare the therapy to radical prostatectomy and could unlock a market that analysts value at $500 million.
By Nick Paul Taylor • Oct. 9, 2024 -
Top medtech conferences in 2025
The lineup includes industrywide conferences and events covering the latest medtech trends in diabetes, orthopedics, cardiac care and surgical robotics.
By Ricky Zipp • Updated Jan. 23, 2025