Cardiac: Page 3
-
J&J to seek FDA approval after small-bore Impella heart pump hits trial goal
The company has predicted the narrower Impella ECP will be easier to insert and implant, as well as enable the use of small bore access and closure techniques.
By Nick Paul Taylor • Oct. 29, 2024 -
Boston Scientific to close Silk Road Medical headquarters, lay off 138 people
Following its acquisition of Silk Road in September, Boston Scientific said it plans to consolidate the company’s work in Minnesota.
By Elise Reuter • Oct. 28, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sarah Silbiger via Getty Images
Sarah Silbiger via Getty Images Trendline
TrendlineMedical device safety in spotlight after high profile recalls
From Philips’ massive recall of respiratory devices to ongoing health risks with breast implants, medical devices tied to patient harm have put a focus on product safety.
By MedTech Dive staff -
Edwards’ TAVR sales faced ongoing hospital constraints in Q3
Hurt by a slowdown in procedures for its heart valve replacement devices, Edwards said it is working with hospitals to manage workflow challenges.
By Susan Kelly • Oct. 25, 2024 -
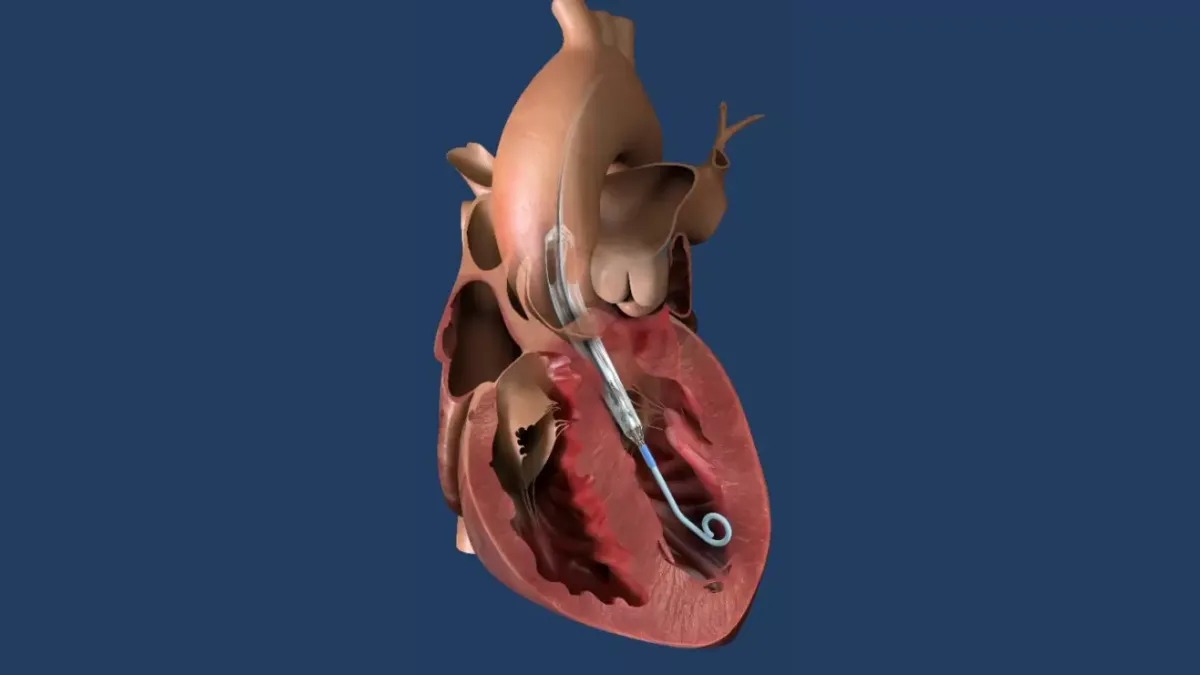
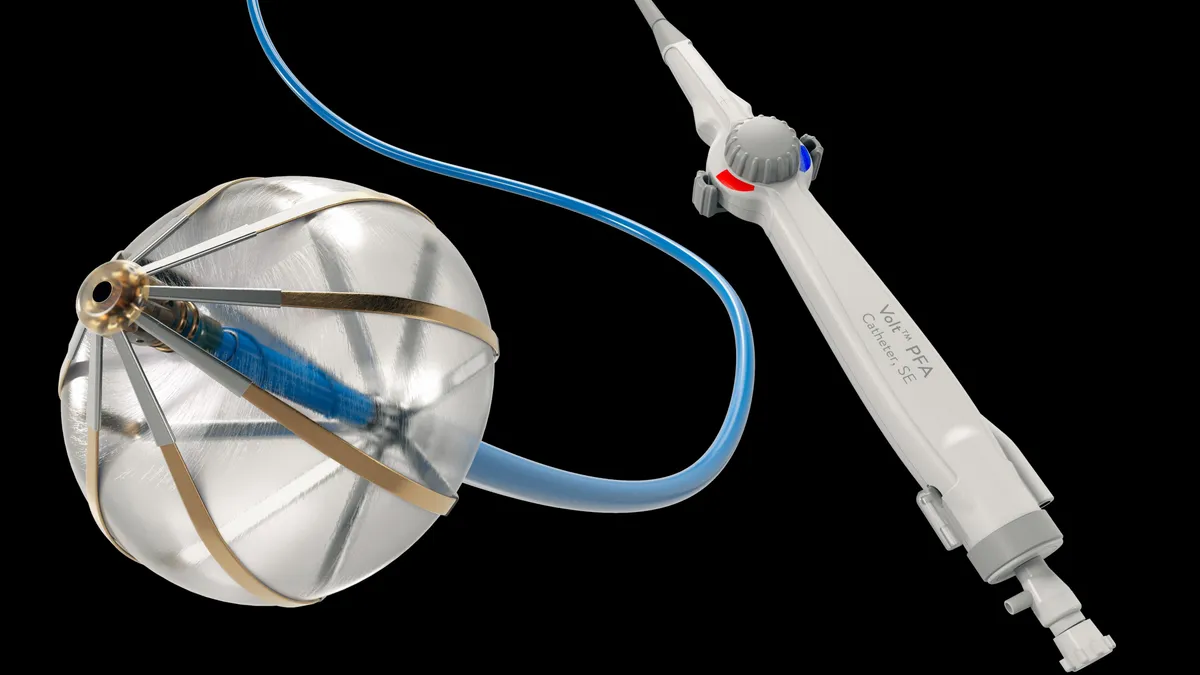
Medtronic wins FDA approval for Affera mapping and ablation system
Affera combines mapping technology with a catheter capable of performing radiofrequency and pulsed field ablation.
By Nick Paul Taylor • Oct. 25, 2024 -
Boston Scientific boosts PFA expectations, citing rapid Farapulse adoption
Demand for the pulsed field ablation device drove the company’s electrophysiology sales up 177% in the third quarter compared to a year ago.
By Susan Kelly • Oct. 23, 2024 -

 Retrieved from iRhythm on October 22, 2024
Retrieved from iRhythm on October 22, 2024
iRhythm’s Zio AT design changes win FDA clearance
The agency cleared one of two 510(k) submissions iRhythm filed for the heart monitor after receiving a warning letter from the agency last year.
By Susan Kelly • Oct. 23, 2024 -
Boston Scientific, Edwards and Dexcom usher in second week of earnings
Johnson & Johnson, Abbott and Intuitive Surgical made a mixed start to earnings season last week.
By Nick Paul Taylor • Oct. 22, 2024 -
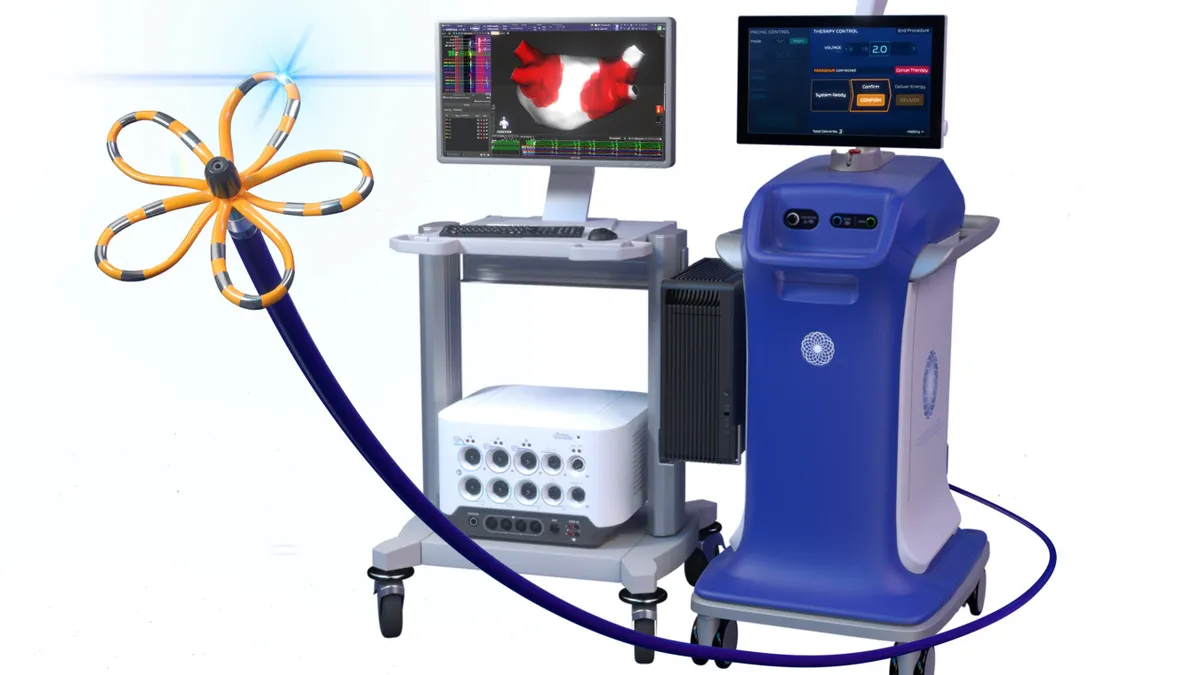
Boston Scientific wins FDA approval for Farapulse cardiac mapping
Stifel analyst Rick Wise said Boston Scientific can now offer “one-stop-shopping” with a pulsed field ablation catheter and integrated mapping system to treat atrial fibrillation.
By Susan Kelly • Oct. 21, 2024 -
Boston Scientific blood-blocking agent tied to additional 2 deaths, 8 injuries
After a February recall, Boston Scientific has warned physicians about new safety risks for Obsidio Embolic, which is now connected to a total of 15 injuries and four deaths.
By Nick Paul Taylor • Oct. 21, 2024 -
J&J’s recent medtech buys help to prop up devices unit
Johnson & Johnson’s medtech acquisitions over the past year have fueled growth for its cardiovascular group, offsetting challenges in businesses like orthopedics and surgery.
By Ricky Zipp • Oct. 15, 2024 -
Medtronic to evaluate Affera in ventricular tachycardia
The FDA approved an early feasibility study of Medtronic’s Affera mapping and ablation system and Sphere-9 catheter in patients with ventricular tachycardia, an abnormal heart rhythm.
By Susan Kelly • Oct. 15, 2024 -
J&J, Abbott and Intuitive kick off latest medtech earnings season
From pulsed field ablation to diabetes tech and surgical robots, the updates will provide an early look at how key medtech markets performed last quarter.
By Nick Paul Taylor • Oct. 14, 2024 -
Abbott enrolls pivotal PFA study 4 months ahead of schedule
The company reported the enrollment milestone alongside news that it received clearance to sell its Advisor HD Grid X Mapping Catheter and started a trial of its Tactiflex Duo Ablation Catheter.
By Nick Paul Taylor • Oct. 11, 2024 -
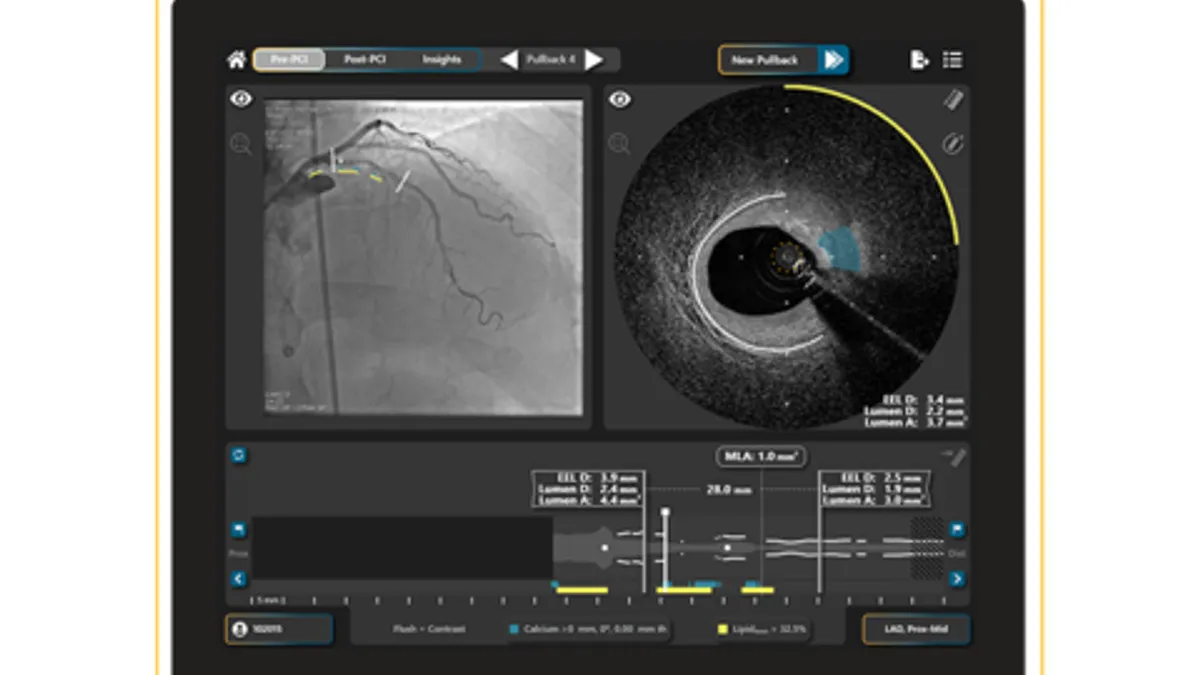
Elucid wins FDA 510(k) nod for heart plaque image analysis software
The software turns coronary CT angiography images into 3D models that quantify and classify plaque morphology to improve predictions of heart attack and stroke risk.
By Nick Paul Taylor • Oct. 4, 2024 -
FDA authorizes Pi-Cardia’s valve-in-valve TAVR device
Pi-Cardia designed the Shortcut device to mitigate the risk of coronary artery obstruction by splitting aortic valve leaflets before valve placement.
By Nick Paul Taylor • Oct. 3, 2024 -
Top medtech conferences in 2025
The lineup includes industrywide conferences and events covering the latest medtech trends in diabetes, orthopedics, cardiac care and surgical robotics.
By Ricky Zipp • Updated Jan. 23, 2025 -
Recalled heart devices had limited clinical testing, study finds
Just 30 of 157 heart devices with Class I recalls underwent premarket clinical testing, according to a study published in the Annals of Internal Medicine.
By Elise Reuter • Sept. 24, 2024 -
Merit Medical inks $210M takeover of Cook’s lead management business
Merit predicts the portfolio, which includes devices used in procedures to remove or replace heart rhythm device leads, will add $40 million a year to its sales.
By Nick Paul Taylor • Sept. 18, 2024 -
J&J leads $50M financing for imaging company Spectrawave
The Series B round will support commercial expansion and product additions for Spectrawave’s system used in the treatment of coronary artery disease.
By Susan Kelly • Sept. 11, 2024 -
European heart group recommends renal denervation for some patients
The European Society of Cardiology said the treatment may be considered for certain patients with uncontrolled, drug-resistant high blood pressure but outlined lingering concerns.
By Nick Paul Taylor • Sept. 4, 2024 -
J&J to buy heart failure implant maker V-Wave for up to $1.7B
J&J has agreed to pay $600 million upfront for the private company as it works to expand its portfolio of cardiovascular devices.
By Susan Kelly • Aug. 20, 2024 -
Edwards spends $1.2B on 2 heart device firms, extending deal streak
Alongside the acquisitions, Edwards reported a slowdown in its core TAVR business in the second quarter that sent its shares tumbling.
By Susan Kelly • July 25, 2024 -
Boston Scientific tips strong growth for Farapulse after successful launch
Questions on Boston Scientific’s pulsed field ablation system once again dominated the company’s earnings call.
By Ricky Zipp • July 24, 2024 -
Q&A
‘Our next frontier is prediction’: Medtronic on AI and heart disease
Stacey Churchwell, who leads the company’s cardiovascular diagnostics and services business, says artificial intelligence is reducing false positives in its insertable cardiac monitors, saving clinicians valuable time.
By Susan Kelly • July 22, 2024 -
Abbott previews PFA, over-the-counter CGM markets
Electrophysiology sales held up against rivals’ launches of pulsed field ablation systems. Meanwhile, Abbott is gauging sales of its first OTC CGMs.
By Elise Reuter • July 18, 2024