Cardiac
-
Boston Scientific unveils plans for new Watchman device
The company hopes to launch its next-generation Watchman system in the second half of 2027 or early 2028.
By Elise Reuter • Oct. 1, 2025 -
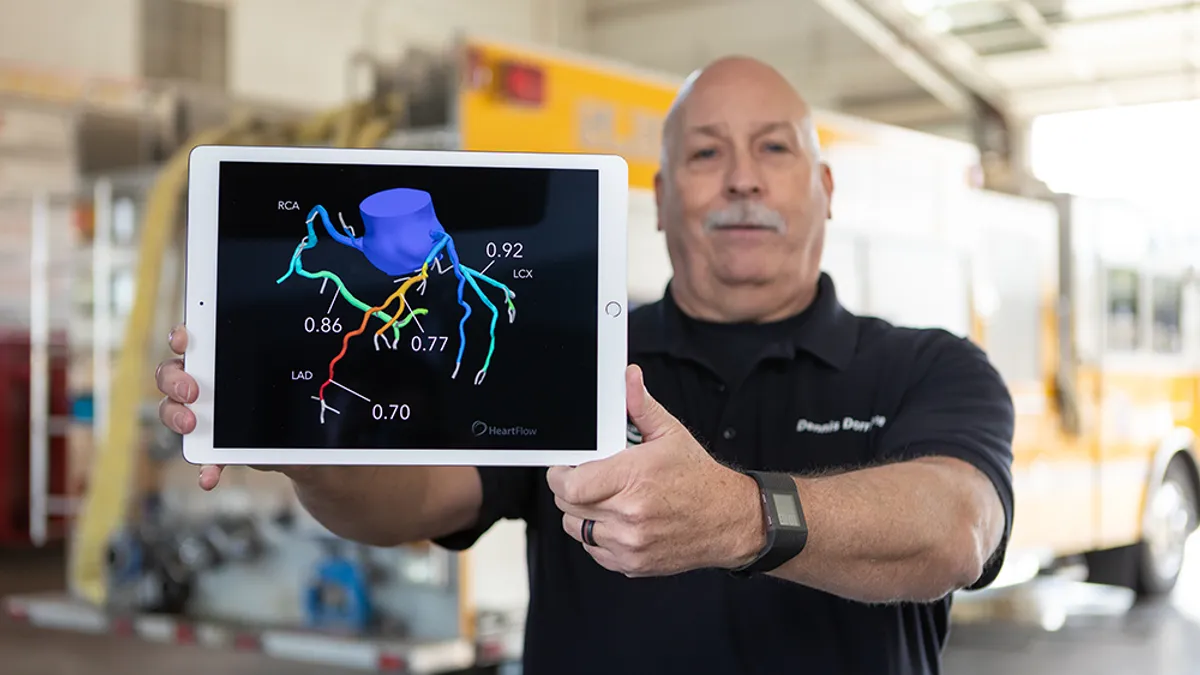
Heartflow receives FDA clearance for updated plaque analysis platform
The updated algorithm shows a 21% improvement in plaque detection, compared to the original version.
By Nick Paul Taylor • Sept. 25, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Cardiosense names Eric Meizlish as CEO
Cardiosense’s switch to a leader with experience scaling healthtech companies comes as it works to build on the recent 510(k) clearance of CardioTag.
By Nick Paul Taylor • Updated Sept. 25, 2025 -
J&J’s Abiomed starts third heart pump controller recall since June
As of Aug. 27, the company had reported five serious injuries and no deaths associated with the issue.
By Nick Paul Taylor • Sept. 24, 2025 -
Profile
How SS Innovations is expanding robotic surgery’s reach
Heart surgeon Sudhir Srivastava saw a global need for less-invasive surgical care at an affordable price. His company, SS Innovations, built a robot that has now been used in more than 5,000 surgeries.
By Susan Kelly • Sept. 22, 2025 -
FDA posts early alert about Abbott’s Tactiflex Ablation Catheter
Catheter tips came off three damaged devices during surgery but none of the patients suffered serious injuries or died.
By Nick Paul Taylor • Updated Sept. 22, 2025 -
J&J launches IVL device in Europe; Medtronic looks at pacing for more patients
J&J’s Shockwave Javelin intravascular lithotripsy catheter treats people with peripheral artery disease. Elsewhere, Medtronic began a pivotal trial to evaluate its pacemakers in a new patient group.
By Susan Kelly • Sept. 18, 2025 -
Boston Scientific inks $88M Elutia deal to challenge Medtronic
BTIG analysts estimate that Elutia’s Elupro and Medtronic’s TYRX are competing for a $600 million opportunity in the U.S. cardiac rhythm management sector.
By Nick Paul Taylor • Updated Sept. 11, 2025 -
Pulse wins IDE approval; Galvanize pulls in $100M
Pulse Biosciences will study PFA in cardiac surgery. Meanwhile, Galvanize Therapeutics also named a new CEO, and PFA pioneer Steven Mickelsen has launched another company.
By Susan Kelly • Sept. 9, 2025 -
Microbot receives 510(k) clearance for endovascular robot
The company will now complete final commercial activities in preparation for targeting the 2.5 million peripheral vascular procedures performed in the U.S. each year.
By Nick Paul Taylor • Sept. 9, 2025 -
Renal denervation gets strong backing from cardiologists ahead of Medicare coverage decision
Doctors told the Centers for Medicare and Medicaid Services that more patients need access to the procedure, with one calling it the start of a new era in hypertension management.
By Susan Kelly • Sept. 4, 2025 -
Kardium receives FDA OK for pulsed field ablation system
The company raised $250 million in July to expand its manufacturing capabilities and establish multiple teams to support the launch.
By Nick Paul Taylor • Sept. 4, 2025 -
3 medtech takeaways from ESC 2025
Pulsed field ablation and transcatheter aortic valve implants were in focus at the European Society of Cardiology Congress in Madrid.
By Susan Kelly • Sept. 3, 2025 -
Edwards, Medtronic and Abbott stand to benefit from new TAVR guidelines in Europe
Analysts said the companies should benefit “as the therapy algorithm is further streamlined and additional patients are encouraged to receive TAVR.”
By Nick Paul Taylor • Sept. 3, 2025 -
J&J removes some Abiomed heart pump controllers from market
Abiomed reported one death associated with a pump driver circuit assembly that does not meet current specifications. The recall affects 69 controllers, a J&J spokesperson said.
By Susan Kelly • Aug. 28, 2025 -
Deep Dive
4 medtech topics to watch for the rest of 2025
From M&A to MDUFA and the developing market in renal denervation, MedTech Dive covers the key issues to watch in the final months of the year.
By Ricky Zipp , Susan Kelly , Elise Reuter • Aug. 25, 2025 -
Boston Scientific recalls carotid stents over manufacturing defect
The FDA said the defect could injure blood vessels, damage the stent or release debris that could travel to the brain and cause a stroke.
By Nick Paul Taylor • Aug. 25, 2025 -
Q&A
How Aktiia built the first over-the-counter cuffless blood pressure monitor
Aktiia received FDA clearance in July for the first cuffless blood pressure monitor authorized to be sold over the counter.
By Elise Reuter • Aug. 20, 2025 -
Medtronic recall of heart vent catheters tied to 3 serious injuries
The FDA said the request to quarantine the devices followed reports of the products “resisting shape retention when being bent.”
By Nick Paul Taylor • Aug. 19, 2025 -
Heart devices pace medtech’s summer funding flows
July and August have brought a steady beat of private financings for young medtech companies, with several deals supporting new technologies to treat heart conditions.
By Susan Kelly • Aug. 18, 2025 -
Reprieve Cardiovascular raises $61M; Conformal Medical nets $32M for LAAO
The fresh funding will support pivotal trials in progress and commercialization preparations at the two heart-focused companies.
By Susan Kelly • Aug. 14, 2025 -
Cardiosense appoints John Martin as chief medical officer
The appointment comes weeks after the company received 510(k) clearance for its wearable heart monitor.
By Nick Paul Taylor • Aug. 13, 2025 -
Heartflow’s IPO raises $364M
The company’s stock rose in its first two days on public markets, closing on Monday up almost 58% over the IPO price amid investor enthusiasm.
By Nick Paul Taylor • Aug. 12, 2025 -
Boston Scientific updates instructions of devices linked to 17 deaths
The update covers devices used in procedures to implant the company’s Watchman heart device.
By Nick Paul Taylor • Aug. 8, 2025 -
Boston Scientific tells users about defibrillator problem linked to deaths, injuries
A company spokesperson said in a statement to MedTech Dive that direct causation between the deaths and calcification of the leads cannot be assumed or confirmed.
By Nick Paul Taylor • Aug. 7, 2025