AI
-
GE HealthCare, Mayo Clinic expand partnership around radiology research
Ben Newton, GE HealthCare’s global head of oncology, said the partnership will shift radiation therapy from a “one-size-fits-all” approach to a personalized method.
By Elise Reuter • Dec. 9, 2025 -
HHS outlines strategy to expand AI adoption
Although the strategy is internally focused to start, the agency said it will collaborate with the private sector and identify “priority” conditions and health issues that could be addressed with AI tools.
By Sydney Halleman • Dec. 8, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sarah Silbiger via Getty Images
Sarah Silbiger via Getty Images Trendline
TrendlineAI tools are transforming the medtech space
As artificial intelligence becomes more prominent in the medtech sector, the technology is changing what it means to be a medical device — and attracting top companies.
By MedTech Dive staff -
PitchBook: AI tuck-in deals to drive M&A acceleration in 2026
Buyers will focus on “tuck-ins that add AI or data-driven capabilities or can meaningfully improve scale against emerging competitors,” the analysts said.
By Nick Paul Taylor • Dec. 3, 2025 -
Lawmakers propose Medicare reimbursement pathway for AI devices
AdvaMed supports the legislation, which is intended to ensure AI-enabled devices receive an accurate CMS payment code.
By Nick Paul Taylor • Nov. 26, 2025 -
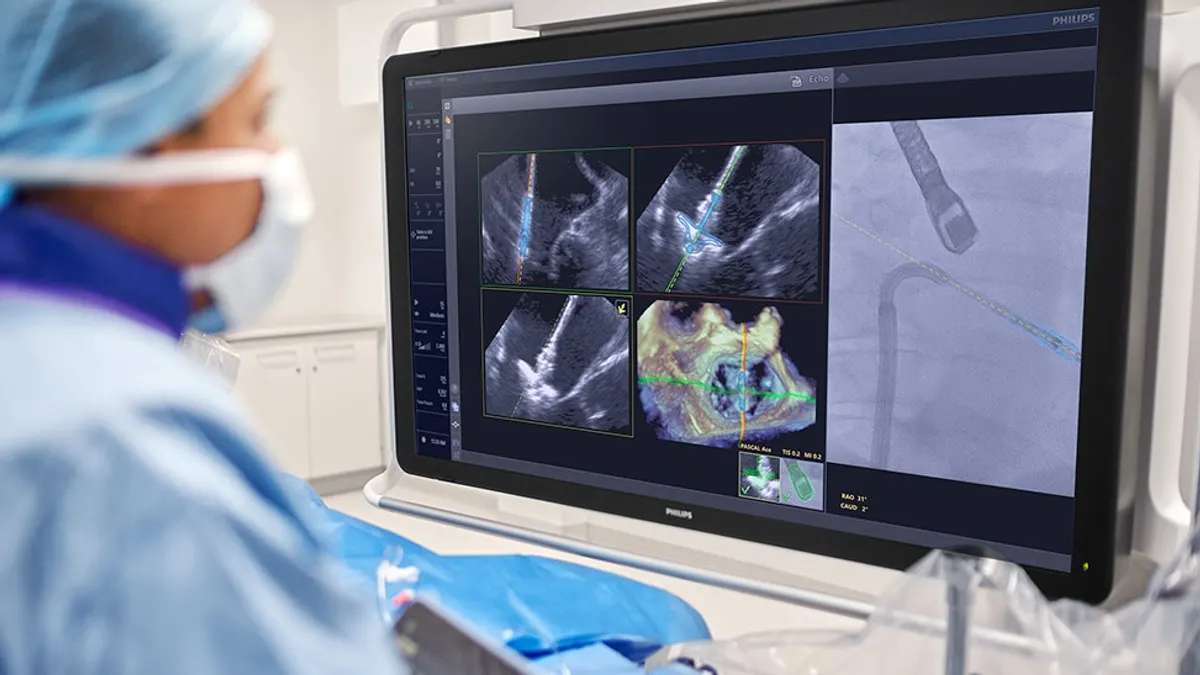
Philips, Edwards team on AI-based guide for mitral valve repair
Artificial intelligence combines ultrasound and X-ray images to give physicians a real-time 3D view of the repair device during transcatheter heart procedures.
By Susan Kelly • Nov. 19, 2025 -
Clairity raises $43M to commercialize breast cancer risk prediction tool
The funding will support reimbursement initiatives and partnerships to make Clairity Breast accessible across community imaging centers nationwide.
By Nick Paul Taylor • Nov. 17, 2025 -
Cornerstone raises $200M to commercialize surgical robot
The financing comes 11 months after Cornerstone raised $70 million to support the launch of its device in China.
By Nick Paul Taylor • Nov. 13, 2025 -
Medtech VC funding slows in Q3: PitchBook
The lowest level of quarterly VC funding since 2023 has tempered expectations that the sector will achieve its best year since 2021.
By Nick Paul Taylor • Nov. 12, 2025 -
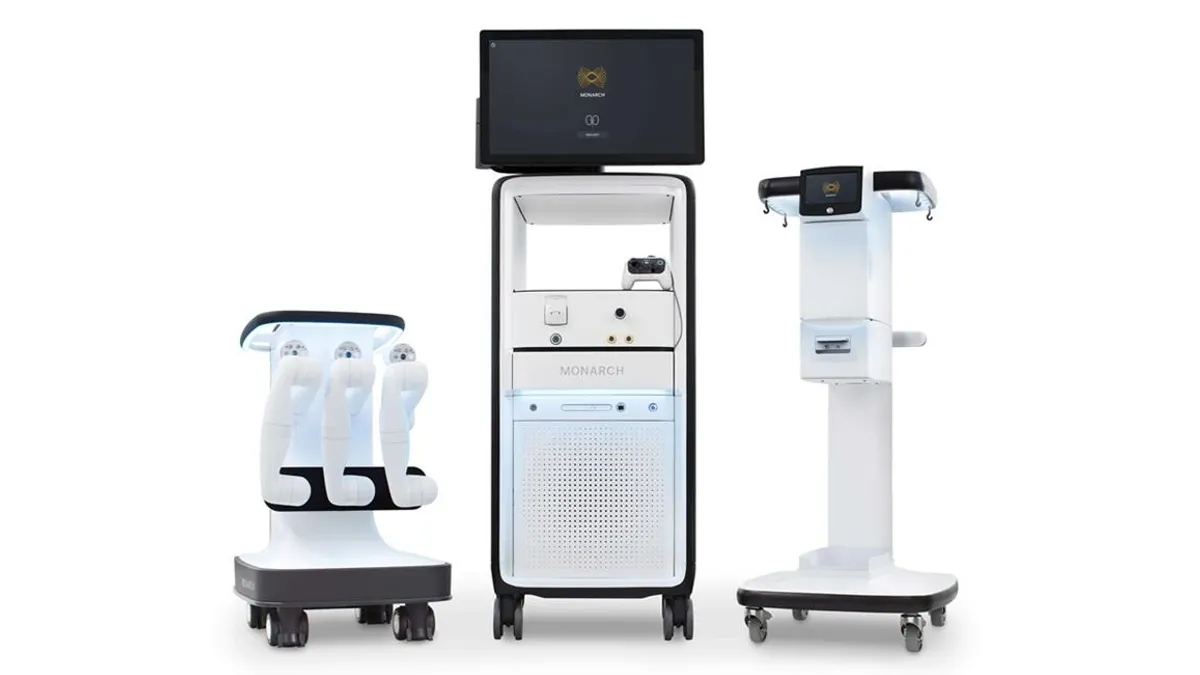
J&J partners with Nvidia on AI for kidney stone procedures
The medtech firm is using Nvidia’s technology in its Monarch robotic platform for urology.
By Elise Reuter • Oct. 28, 2025 -
Olympus execs talk about import alert, GI robot and AI
CEO Bob White and Chief Strategy Officer Gabriela Kaynor discussed the medtech company’s quality and technology priorities after a year of change.
By Elise Reuter • Oct. 28, 2025 -
Deep Dive
Health systems are racing to adopt AI. But can they prove its value?
Measuring financial returns from AI can be challenging, experts said at the HLTH conference last week. But other metrics, like provider and patient satisfaction, are important too — and also impact providers’ bottom lines.
By Emily Olsen • Oct. 28, 2025 -
J&J awards first grants from AI surgery fund
Mayo Clinic and QAS.AI will receive funding and mentorship to help advance their projects.
By Elise Reuter • Oct. 27, 2025 -
5 AI takeaways from AdvaMed’s conference
Medical device firms discussed privacy, regulations and prioritizing projects as AI becomes more prevalent in the industry.
By Elise Reuter • Oct. 14, 2025 -
Intuitive expands AI in Ion; Horizon performs first robotic cataract surgery
The FDA cleared AI and imaging updates for Intuitive’s lung biopsy robot. Elsewhere, Horizon completed the first cataract surgery with its robotic system, and MMI began a neurosurgical study.
By Susan Kelly • Oct. 10, 2025 -
How 4 medtech CEOs are using AI
At AdvaMed’s The MedTech Conference, executives said they’re incorporating AI in more devices, but figuring out how to use the technology internally is challenging.
By Elise Reuter • Oct. 7, 2025 -
Q&A
Hospital for Special Surgery’s Ashis Barad says AI must solve real problems
Barad, chief digital and technology officer at HSS, outlined his approach to evaluating AI tools and how the orthopedic hospital uses the technology.
By Elise Reuter • Oct. 3, 2025 -
CDRH shares regulatory guidance priorities for the coming 12 months
Use of real-world evidence to support regulatory decisions and predetermined change control plans for medical devices are two areas of focus for the agency.
By Nick Paul Taylor • Oct. 2, 2025 -
Deep Dive
AI could transform healthcare. Can safety-net providers keep up?
Implementing artificial intelligence requires significant human labor and technical expertise, threatening to create a digital divide between highly resourced health systems and safety-net providers.
By Emily Olsen • Oct. 2, 2025 -
FDA seeks feedback on monitoring real-world performance of AI devices
The agency is looking for ways to detect, assess and mitigate changes to the performance of AI-enabled devices over time.
By Nick Paul Taylor • Oct. 1, 2025 -
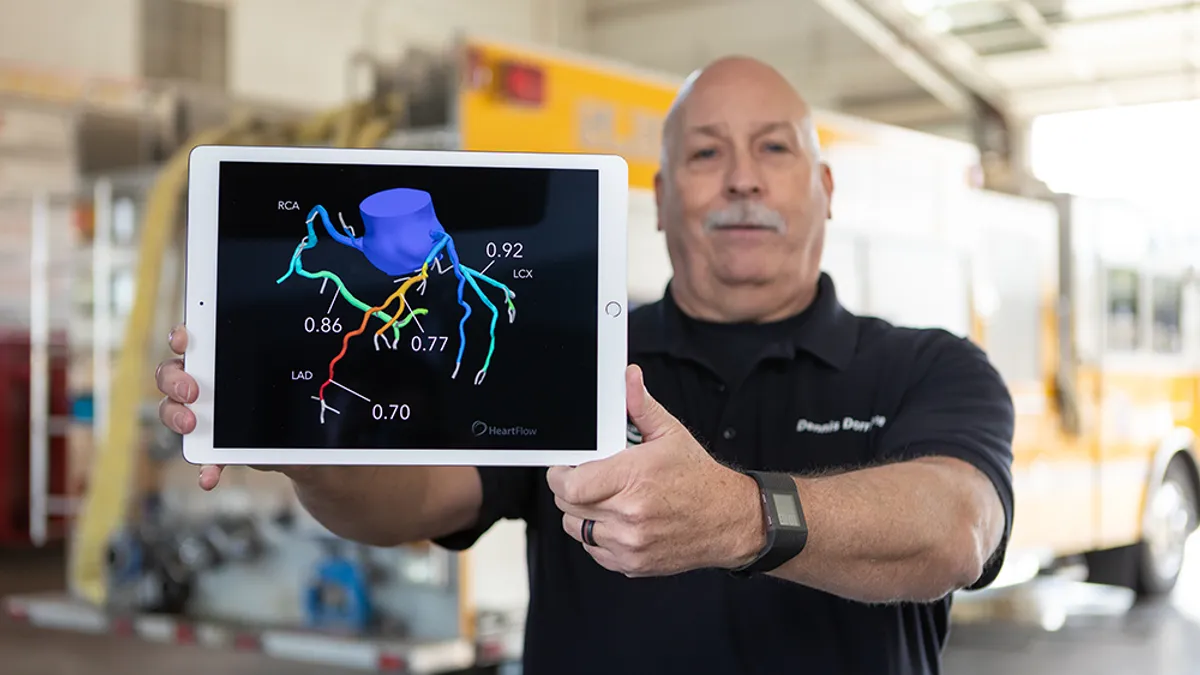
Heartflow receives FDA clearance for updated plaque analysis platform
The updated algorithm shows a 21% improvement in plaque detection, compared to the original version.
By Nick Paul Taylor • Sept. 25, 2025 -
Deep Dive
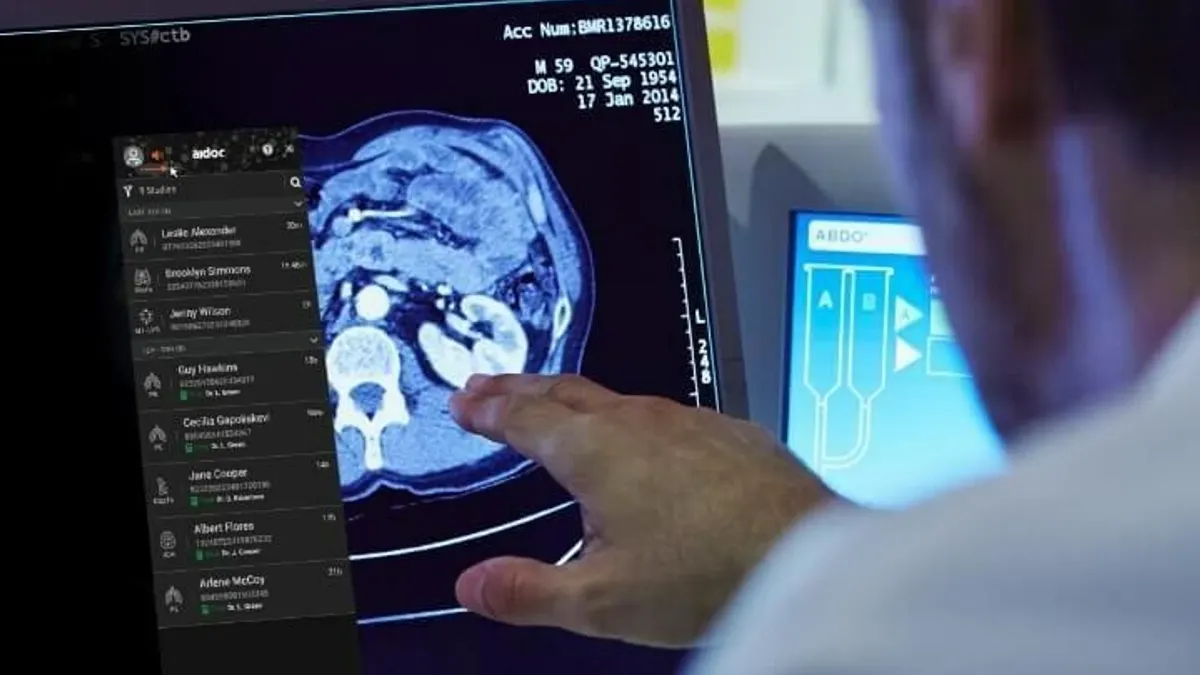
How three companies are using foundation models in radiology
Some firms, such as Aidoc, are working directly with the FDA. Others, such as HOPPR, are offering their foundation models for medtech companies to build their own AI tools.
By Elise Reuter • Sept. 16, 2025 -
Deep Dive
Foundation models are medtech’s newest trend. But what are they?
The AI models, which are built on massive datasets and can be adapted to multiple tasks, have become a part of medtech companies’ lexicon in the past few years.
By Elise Reuter • Sept. 15, 2025 -
AI drives medtech investment in 2025
Medical device companies working with artificial intelligence raised funding and were acquisition targets in recent months.
By Elise Reuter • Sept. 12, 2025 -
GE HealthCare inks Icometrix buyout to acquire brain MRI software
The deal includes AI-powered software for detecting known side effects of recently launched Alzheimer’s drugs.
By Nick Paul Taylor • Sept. 11, 2025 -
AI firm Ketryx raises $39M, adds former Medtronic CEO as investor
The company, which helps medtech companies with AI compliance, has raised more than $55 million to date.
By Elise Reuter • Sept. 5, 2025