Dive Brief:
- ZimVie has won U.S. Food and Drug Administration approval for a modified version of its Mobi-C spinal implant.
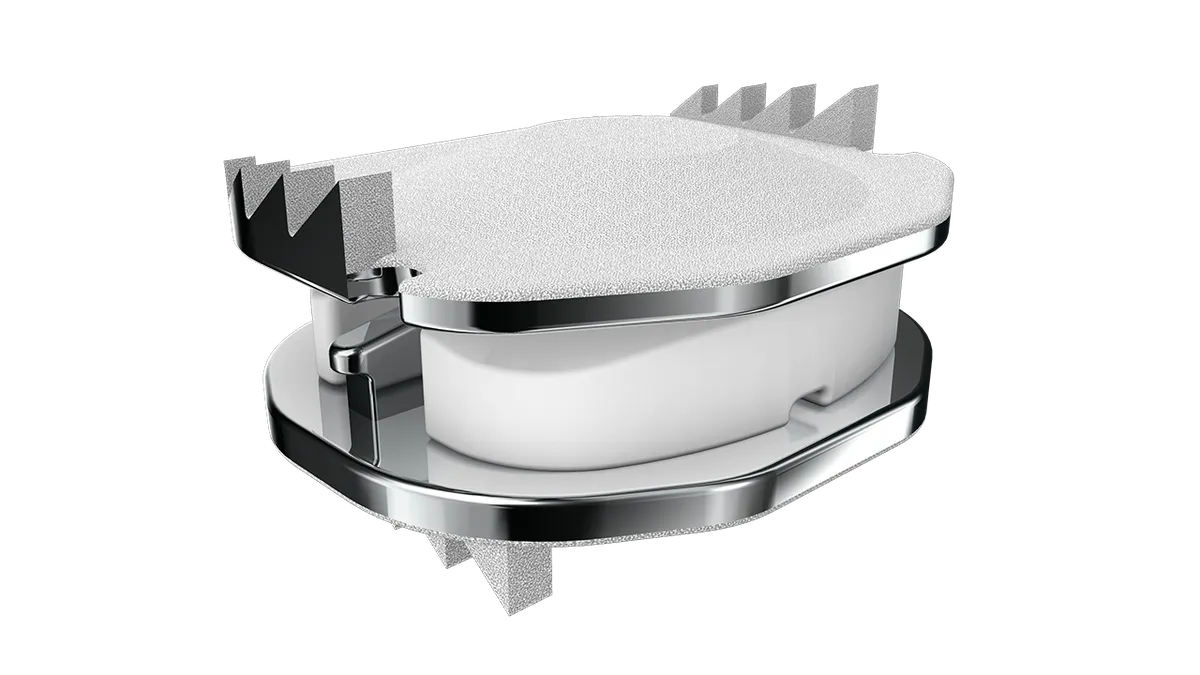
- The recently authorized cervical disc has a smaller height, 4.5mm, than existing versions of the device, which was first approved in the U.S. in 2013. ZimVie said the new product will “address the anatomical needs of the U.S. patient population.”
- Approval of the 4.5mm device follows a good quarter for Mobi-C. ZimVie cited the product as a driver of growth outside the U.S. and a bright spot for a U.S. business that is under competitive pressure in an earnings call last week.
Dive Insight:
Mobi-C was the first cervical disc approved in the U.S. to treat more than one level of the cervical spine. The device outperformed fusion at seven years for two-level cervical disc replacement and has become an important product for ZimVie.
Under the original FDA approval, LDR Spine, a business that Zimmer Biomet bought in 2016, received authorization to provide Mobi-C in three heights, 5mm, 6mm and 7mm. The authorization also covered multiple footprints. ZimVie’s latest premarket approval supplement covers inserts that are 4.5mm high in seven footprints.
“Improper sizing of an artificial disc can lead to problems such as prosthesis migration, subsidence, and segmental kyphosis. We also found from our biomechanical study that increasing the height of an artificial disc by just 1mm reduced the range of motion at that level by around 50%,” Kee Kim, professor and chief of spinal neurosurgery at the University of California Davis, said in a statement from ZimVie. “Even a 5mm height artificial disc may be too tight for some patients.”
ZimVie secured the FDA approval using real-world clinical evidence from European studies. The studies in Europe, where physicians have used Mobi-C since 2004, showed the long-term safety and efficacy of the smaller disc.