Dive Brief:
- Withings has received 510(k) clearance for a six-lead electrocardiogram (ECG), positioning it to launch a smart scale that checks for atrial fibrillation (AFib) in the U.S.
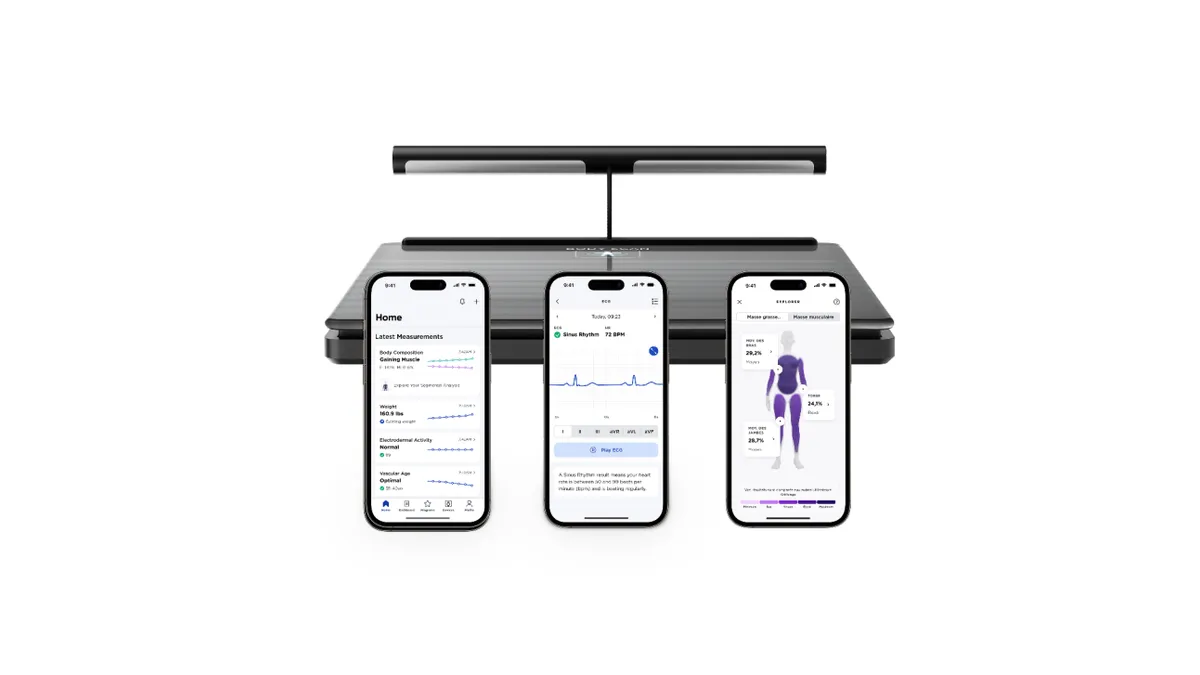
- The French manufacturer of digital health devices has incorporated the ECG into its Body Scan Connected Health Station, a scale that records the electrical signal from the heart, assesses the number of calories the body burns at rest and tracks other health variables.
- With the U.S. Food and Drug Administration clearance removing a regulatory barrier, Withings plans to start selling the device for about $400 next month.
Dive Insight:
Withings already sells Body Scan in other parts of the world, including the U.K., but needed the FDA’s signoff before launching the device in the U.S. The 510(k) clearance covers Withings Scan Monitor 2.0, the regulatory name for Body Scan when the ECG function is activated. Withings Scan Monitor 1.0 used a wrist-worn wearable, ScanWatch, as a one-lead ECG.
Users can record an ECG by stepping onto the scale and holding a handle. In theory, recording ECGs as part of a daily weigh-in and health check could accelerate the diagnosis and treatment of AFib, a disease that puts people at higher risk for stroke and heart failure. Wrist-worn wearables such as Apple Watch and Withings’ own ScanWatch record ECGs, but only use a single lead. Dutch researchers found a six-lead ECG, KardiaMobile 6L, had better sensitivity and specificity than single-lead wearables.
Withings compared its ECG scale to a 12-lead reference device in a clinical trial that is cited in the 510(k) paperwork. The clinical trial assessed sensitivity and specificity in detecting AFib using both devices.
While the device is recording the weight and electrical heart activity of the user, it can also measure fat and muscle mass in different parts of the body, monitor the speed at which heart waves move along the arteries to assess cardiovascular health, and check small nerve activity.