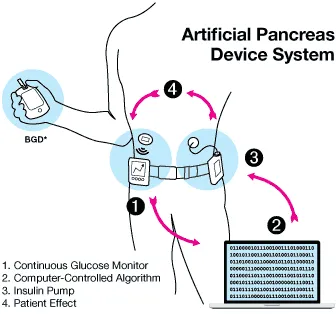
For decades, researchers and diabetes patients have been working to develop an artificial pancreas with the ability to detect blood sugar levels and administer insulin as needed. Those efforts have led to the commercialization of the first hybrid closed-loop systems —where continuous glucose monitors and insulin pumps work in tandem — in the multibillion-dollar market for diabetes devices.
These systems still come with some limitations. For one, patients can’t choose which devices they want to pair together as the software that lets the two devices work together currently is integrated into insulin pumps.
So as companies including Medtronic, Dexcom and Tandem Diabetes Care worked to build their own connected devices, people with diabetes and family members started grassroots efforts to build their own systems, including an app called Loop that can be used to manage dosing with devices of a person’s choosing.
Building on that work, a non-profit company called Tidepool ran a study of people who used the DIY Loop app and brought a version of the system to the Food and Drug Administration.

“A bunch of us, my daughter included, were actually users of DIY Loop, starting in about 2016,” Tidepool CEO and Founder Howard Look said in an interview. “And we really loved it. It was a really wonderful way to manage diabetes and really helped reduce the burden of day-to-day management of Type 1” diabetes.
The resulting product, Tidepool Loop, received FDA clearance in January, creating an option for people to choose the pumps or CGMs they want to pair. That comes with a caveat: while some CGMs currently are able to pair with Tidepool, no insulin pumps have been approved to pair with third-party controllers.
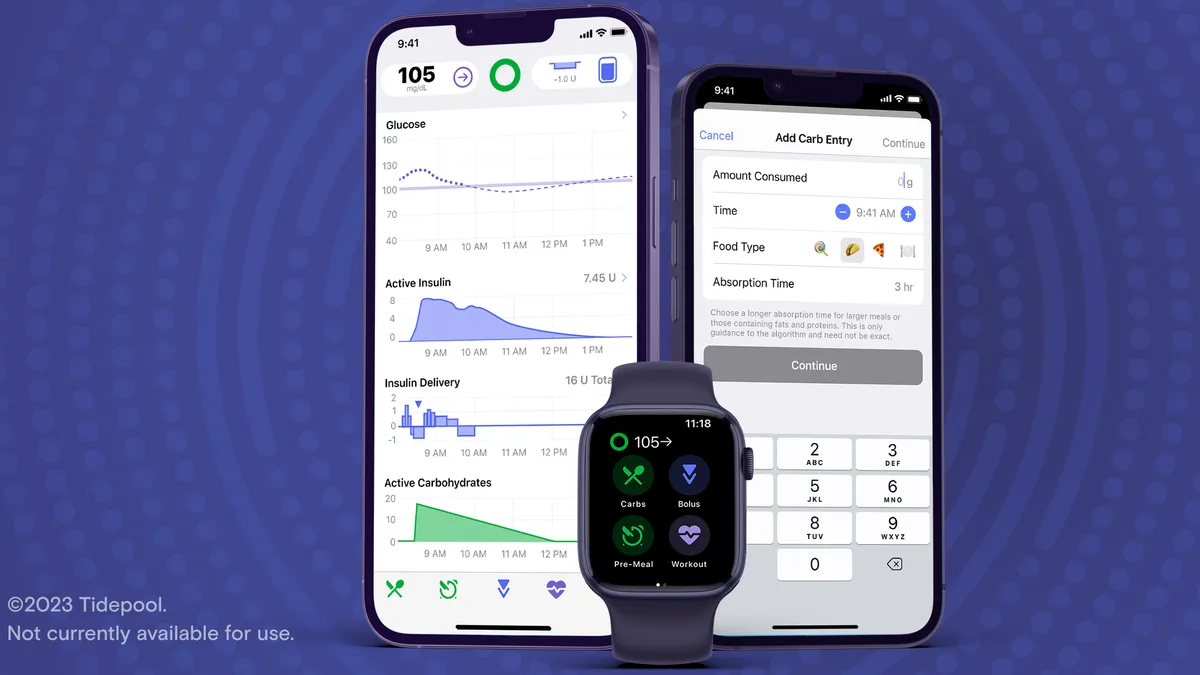
The app is designed to predict a person’s blood sugar levels and ensure they receive the correct dose of insulin. It also has a feature for users to adjust their mealtime insulin dose by entering the estimated amount of carbohydrates they plan to eat.
Tidepool is smaller than other diabetes companies in the market. Abbott Laboratories, which hopes to make its CGMs a $10 billion product in the next five years, had annual revenue of $43.65 billion in 2022 and insulin pump-maker Tandem, which plans to grow its installed base to 1 million people by 2027, had revenue of $801.2 million. Tidepool Loop had $8.06 million in revenue in the 12 months ended in June 30, 2021 and had $7.5 million in assets at the end of the year, according to its most recent tax filing.
Still, the company represents a rare example of a patient-driven device going through the FDA’s regulatory process and could clear a regulatory pathway for future device-agnostic automated insulin dosing apps.
“Type 1 diabetes, in particular, is such an individual disease. There's a lot of factors that come into play when managing Type 1, and so having the ability to pick the components that work best for you in your daily management I think is really important and really groundbreaking for the community,” said Marjana Marinac, senior director of regulatory affairs for JDRF, formerly the Juvenile Diabetes Research Foundation, which is focused on Type 1 diabetes.
DIY roots
Early DIY efforts came out of the first DiabetesMine D-Data ExChange at Stanford University in 2013 — a meeting of diabetes advocates and developers — where attendees including Look coined the slogan #WeAreNotWaiting.
It started with patients and family members figuring out how to download data from CGMs to their phones, and a parent creating a device to connect his daughter’s insulin pump to an iPhone. From there, a software engineer named Nate Rackyleft developed the original DIY Loop, an open source app to automate insulin delivery.
“That’s how it evolved. Different people jumped in and started doing different aspects of it,” said Anna McCollister, an independent consultant on data use, access and government and who has Type 1 diabetes. “I think it’s the most compelling and exciting thing that’s happened in digital health.”
Look founded Tidepool as a nonprofit in 2013 to create open-source tools to help people better manage their diabetes. That started with a platform for patients to upload data from multiple devices, including their CGMs and insulin pumps, in one place.
In 2018, the nonprofit group hired some of the people who worked on Loop to develop an FDA-cleared version of the app.
The nonprofit organization and members of the DIY community also worked together to recruit people for a study of patients who used DIY Loop to manage their diabetes. More than 1,000 people stepped up to participate, providing enough data to lead to an FDA submission in 2020. Tidepool worked with backers including the Leona M. and Harry B. Helmsley Charitable Trust and JDRF, who together contributed $6 million to the efforts.
“The base code for Tidepool Loop was developed by the crowd and put on GitHub as open source, and then Howard and his team took that basic code, built on top of it, and set up some regulatory controls and things like that,” McCollister said. “That to me is a milestone, and it's an important one. I think FDA should be applauded for their willingness to do this and to work with a patient-driven effort and nonprofit.”
Tidepool Loop currently is cleared for people with Type 1 diabetes, ages 6 and older. As for people with Type 2 diabetes, Look hopes to expand indications for the device in the future.
What matters to patients?
For Marinac, one important feature is compatibility with Apple products; it’s the first insulin-dosing app that can work on the Apple Watch.
“I use Apple devices currently. So it’s something I think about when I have to get another sort of device to be able to control my insulin dosing,” she said. “What can I use to reduce the number of things I may have to carry around?”
McCollister says she would use Loop over some of the other available systems.
“I feel far more comfortable with the algorithms and error reporting methods that are done through Loop,” she said. “Pretty much everybody I know who's involved in the process is either a patient or the parent or spouse of a patient. So everybody involved in the process has skin in the game,” and a vested interest in fixing bugs quickly, she added.
Another important aspect is which devices will be compatible with Loop at launch.
Insulin pump makers Insulet and Medtronic were development partners, but they are not staying on as launch partners. Medtronic discontinued work on an insulin pump system that would be compatible with Tidepool Loop, and Insulet currently is focused on commercializing its new Omnipod 5 automated insulin delivery system, the companies said in a statement. Users of Tidepool Loop will need to wait for a compatible pump to be cleared by the FDA to work with third-party controllers.
Currently, the only interoperable pumps on the market are cleared to work with controllers made by the same manufacturer, although third-party interoperable pumps are in development, according to Tidepool.
The nonprofit group is still working with CGM-maker Dexcom and is in “active development” with an alternate controller enabled (ACE) pump manufacturer, “a company that people know and love,” Look said. Because Tidepool’s device was cleared to allow for third-party interoperability, “It’s very straightforward for us to add new CGM and new ACE pump devices over time without a submission,” he added, once they are approved by the FDA.
Next steps
The Tidepool Loop app soon will be available for iPhone on Apple’s App Store, although it will require a prescription, Look said. Physicians can write the prescription with personalized settings they think would be a good starting point for the patient, who will then get a prescription code via email or text message to download the app.
The company hasn’t yet set pricing and will work with device-makers to do that, Look added.
“Part of our mission is to spread our tech as broadly as we can. That means keeping the cost as low as we can,” the CEO said.
In 2022, the organization had $2 million in cash and accounts receivable, and plans to use the revenue from its services — in addition to donations and grants — to fund its work, according to Tidepool’s financial statements.
“I believe that there will always be a vibrant, thriving DIY community,” Look said. “[This] provides an option for people who don't want to build their own system or want the experience that Loop provides, but also want the reassurance of using something that is prescribed by their physician and has FDA clearance.”