Dive Brief:
- FDA awarded marketing authorization Thursday to a first-of-its-kind, fast-acting diagnostic test intended to help detect infection around a joint replacement. The condition is traditionally assessed using X-ray imaging or laboratory analysis, the agency said.
- The De Novo clearance went to Claymont, Delaware-based CD Diagnostics, which Zimmer Biomet acquired in 2016.
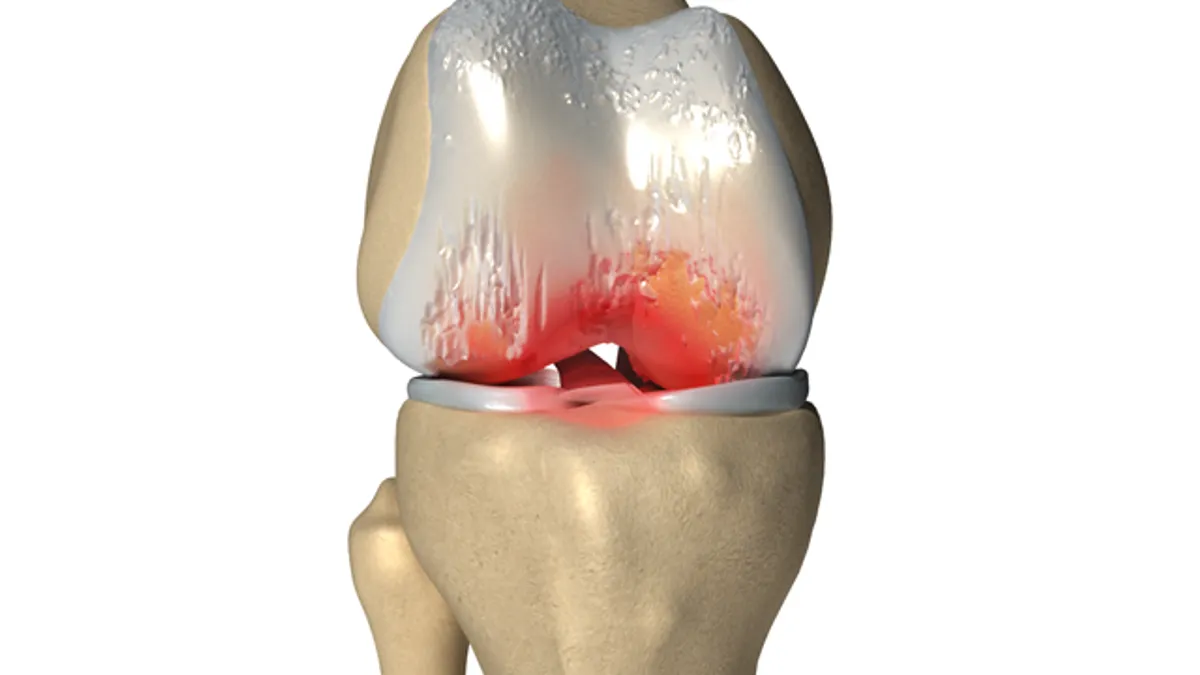
- The Synovasure Lateral Flow Test Kit is designed to identify presence of certain antimicrobial proteins in fluid surrounding an implant within 10 minutes. In a clinical study, the product helped flag indicators of infection in about nine out of 10 patients deemed to have infection.
Dive Insight:
There are more than one million total joint replacement procedures in the U.S. each year, and the American Academy of Orthopaedic Surgeons estimates about 1% of those patients will develop periprosthetic joint infection. Infections can develop during initial surgery recovery or years after receiving the implant.
The in vitro test is meant for use in patients being considered for revision surgery to replace an implant or correct for complications. The product analyzes the sinovial fluid surrounding an implant and aims to discern presence of antimicrobial proteins called alpha defensins, which are activated by white blood cells in the case of infection.
CD submitted data from a study of 305 synovial fluid samples from patients with a total knee or hip replacement being evaluated for revision surgery. FDA said in 89.5% of cases deemed to have an infection diagnosis by standard of care criteria, the Synovasure test identified the presence of alpha denfensin.
"Whereas before surgeons may have opted for surgery when the presence of an infection was unclear, with this test, they have more information and could potentially reduce patient risk by avoiding unnecessary revision operations for replacement joints," said Tim Stenzel, director of the Office of In Vitro Diagnostics and Radiological Health at FDA, in a statement.
The Synovasure test is not indicated to identify a specific type of infection, and findings are meant to be supplemented by additional clinical and diagnostic metrics to form a diagnosis, FDA said.
The agency will regulate the test as a Class II device. The De Novo clearance establishes the category, "Device to detect and measure non-microbial analytes to aid in the detection and identification of localized human infections."