Dive Brief:
- Tasso has received 510(k) clearance for its blood lancet, positioning it to market a device that is intended to help meet demand for remote testing services.
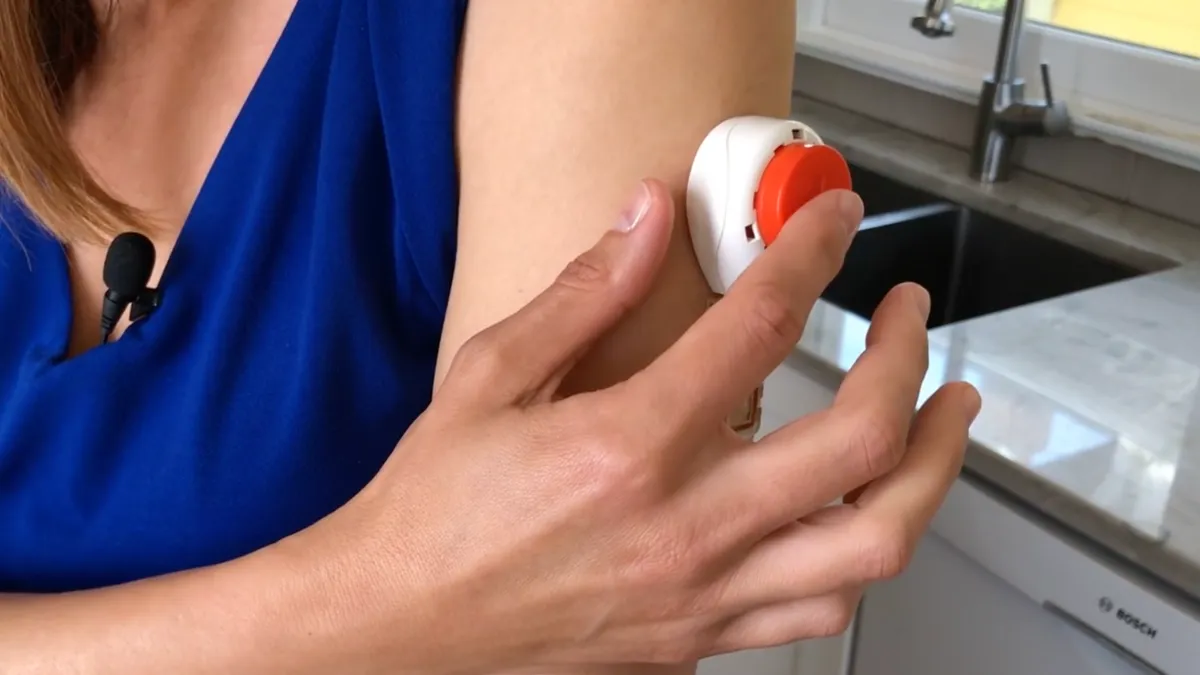
- The product, Tasso+, is designed to enable the collection of blood at home. After sticking the device to the arm, users press a button to release a lancet and initiate the collection of blood.
- Tasso, which raised a $100 million series B financing round last year, is pitching the device as an enabler of decentralized clinical trials because it could simplify the collection of blood at home to support pharmacokinetic and biomarker research endpoints.
Dive Insight:
Tasso is launching the device into a changing lancet market. Late last year, the Food and Drug Administration published a final rule to reclassify three types of lancets, including single use only blood lancets with an integral sharps injury prevention feature, from Class I to Class II. The change imposed special controls on the lancets for the first time.
Because Tasso+ is a single use only blood lancet with an integral sharps injury prevention feature, it is subject to the Class II special controls. Tasso’s application for 510(k) clearance states the device complies with the special controls imposed by the FDA.
The FDA has cleared 12 devices with the same product code as Tasso+, indicating they are single use only blood lancets with integral sharps injury prevention features, since it published the final rule last year. However, Tasso claims its device is the first “single-use, patient-centric blood collection product” to get a Class II clearance as part of the new reclassification process for lancets.
Tasso secured clearance on the strength of its compliance with the special controls and FDA-recognized consensus standards, and in light of its clinical performance data. Researchers reported a 94.2% success rate when subjects used Tasso+ to self-collect blood samples.