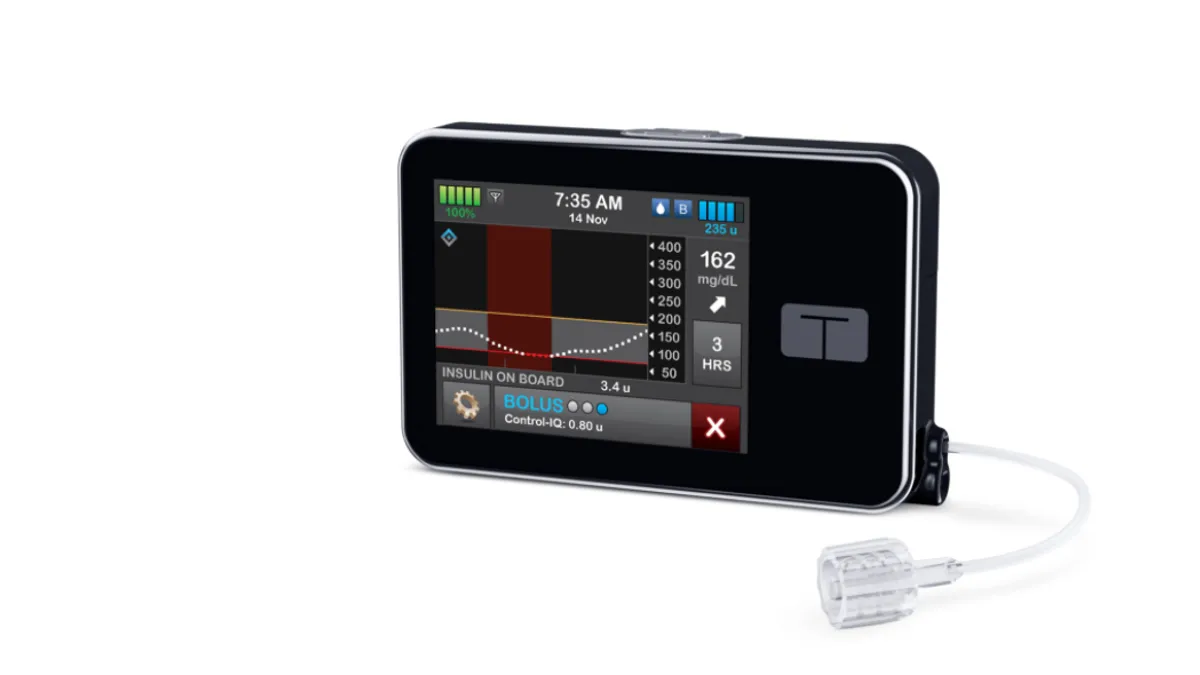
Dive Brief:
- Tandem Diabetes announced Wednesday its insulin dosing algorithm can now be paired with its insulin pump in children as young as 6 years old.
- The age indication expansion, down from a minimum FDA-cleared age of 14, was supported by a six-month, NIH-funded study of children between the ages of 6 and 13 whose time in range on the Control-IQ system was 67%, compared to 53% among individuals in a control group.
- The company launched Control-IQ in the 14-and-up population in January after it won De Novo authorization from FDA as the first interoperable automated glycemic controller at the end of 2019. The algorithm currently integrates with Tandem's pump and Dexcom's G6 continuous glucose monitor. Some 40,000 Tandem pump users have started using the algorithm since it launched, the company reported.
Dive Insight:
Historically, it's been challenging to incentivize device makers to complete the testing necessary to support a pediatric indication for a product, particularly when it comes to young children. This reality means it's relatively common for care providers to resort to off-label use of devices designed for adults.
But when it comes to the 1.6 million Americans living with Type 1 diabetes, in which 27% of the diagnoses of the chronic condition happen between the ages of 5 and 9, and 33.5% of cases are identified between the ages of 10 and 14, the argument for securing a pediatric indication is compelling.
Blood sugar highs and lows can be especially challenging for a young child to manage. Tandem presented pediatric data from an NIH-backed study at the International Conference on Advanced Technologies and Treatments for Diabetes in February, and the difference in outcomes between those with and without Control-IQ was most significant during the overnight period. Children in the 6 to 13 age group stayed in range 80% of the time versus 54% among their control group counterparts, who were also on a sensor-augmented pump.
Although the system is now indicated for use in patients as young as 6 years old, the label specifies that the technology should not be used in small patients who weigh less than 55 pounds or require less than 10 units of insulin per day.
Over the weekend at the American Diabetes Association's virtual Scientific Sessions, Tandem presented a pair of posters with real-world data on patients' experience with Control-IQ since it launched in the 14-and-older population at the start of this year.
One retrospective analysis revealed that a cohort of more than 1,600 users on Control-IQ for at least 30 days had 78% median time in range. Another dataset looked at different glycemic outcomes among Control-IQ users with Type 1 and Type 2 diabetes. The company analyzed nearly 2,900 Type 1 users on Control-IQ for at least 14 days and saw a 9% increase in median time in range. Among 144 Type 2 users, that increase was 6%.