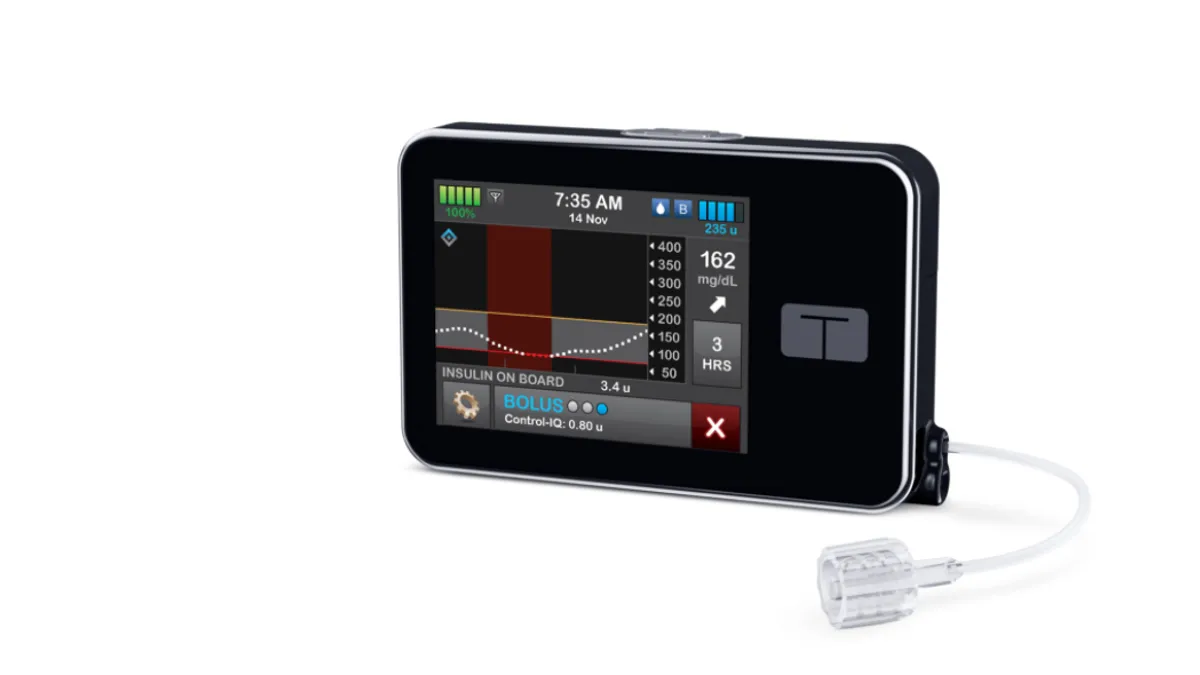
Tandem Diabetes Care, a leading insulin pump provider, expects curbed sales growth in the second quarter amid “pressures” linked to a planned new product launch in the U.S. by rival device maker Medtronic.
The company remains “confident” it will achieve “key goals for this year, both operationally and commercially,” CEO John Sheridan said in Tandem’s first-quarter earnings statement on Wednesday, although Tandem expects Medtronic’s recently approved rival MiniMed 780G device to have a short-term impact on sales.
“We've been competing against that product now in [outside the U.S.] countries for many quarters. Once the product does come to market, ... we expect them to step up promotion. But we think we have a better product. And when you look at the new device, it's certainly a better device, but it's still the same form factor, and it has a sensor that requires fingersticks,” Sheridan added in a conference call with investors.
Brian Hansen, Tandem’s chief commercial officer, said there have been “pressures associated with the Medtronic’s new product launch in the U.S., which created noise and delayed decision-making.”
CFO Leigh Vosseller said on the call that the company expects those pressures to affect second-quarter sales growth. While Tandem forecasts revenue to climb in the period, the increase over the first three months of the year may be smaller than in previous years.
“The bottom end of the [historical] range has been a low double-digit increase over Q1, which is a good starting point for how to think about this year,” Vosseller said.
Shares in Tandem fell 8.4% to $36.58 in Thursday morning trading.
CGM integration
Vosseller’s forecast reflects the impact of the launches of MiniMed 780G, Tandem’s own upcoming smaller Mobi pump and continuous glucose monitors (CGMs). With Tandem still working with Dexcom to integrate its Control-IQ technology with the new G7 CGM, the CFO said people “might be waiting for G7 to be available on the pump.”
That could delay sales until later in the year. Sheridan said on the call that the “launch will scale across the upcoming months, followed by our international rollout.”
Tandem also is working to integrate its technology with Abbott’s FreeStyle Libre 2 and Libre 3 sensors with a view to a “scaled launch in the U.S. in the third quarter,” Sheridan said. Tandem sees the Libre integration as a key opportunity.
“There's approximately 300,000 people who have Type 1 [diabetes] in the U.S. that use the Abbott sensor. ... If we even get 30% or 35% penetration with that community, it's 100,000 people that are potential candidates for pump therapy. So I think that there is a big opportunity with the Abbott device,” the CEO said.
Forecast:
Tandem reiterated its non-GAAP full-year sales target of $885 million to $900 million.