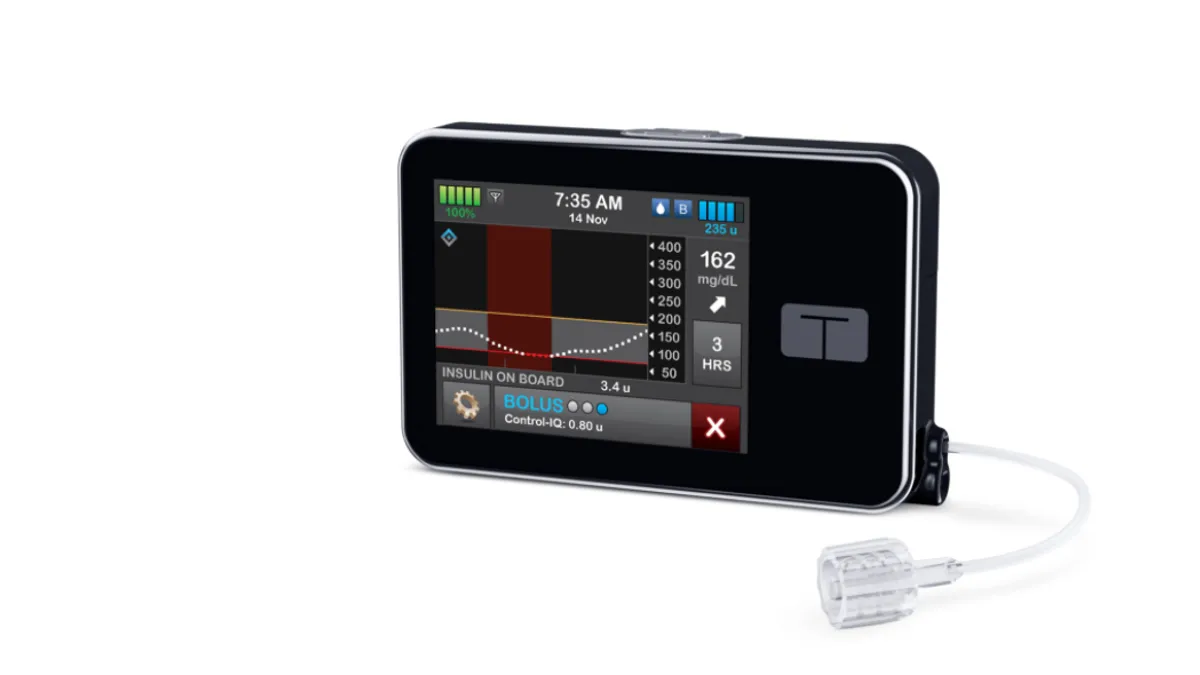
Dive Brief:
- People with Type 2 diabetes spent 3.6 hours a day longer in the target blood glucose range after switching to Tandem Diabetes Care’s t:slim X2 insulin pump with Control-IQ technology in a small clinical trial.
- The study, which Tandem presented at the Annual Diabetes Technology Meeting, enrolled 30 adults with Type 2 diabetes from three clinical centers. Participants used Tandem’s device for six weeks.
- Tandem has identified Type 2 diabetes as one of six growth drivers for its devices, noting that 5% of U.S. patients with the condition use pumps and outlining plans to seek authorization of its Control-IQ technology.
Dive Insight:
In preparation for an attempt to expand into the Type 2 market, Tandem sponsored a clinical trial that evaluated the effect of switching patients from multiple daily injections or basal insulin only to its t:slim X2 insulin pump with Control-IQ technology. Control-IQ automatically adjusts basal insulin based on data from a compatible integrated continuous glucose monitor.
After tracking patients on their original regimens for two to four weeks, the investigators gave all the participants the Tandem technology and a Dexcom G6 continuous glucose monitor. The study tracked the effects of the Tandem technology over six weeks.
Over that period, mean time in the 70 to 180 mg/dL target range improved by 15 percentage points, the study found. The change reflected similar improvements in both patients who previously took multiple daily injections and prior basal insulin only. Tandem saw no increase in CGM-measured hypoglycemia. Median time in closed loop was 96% and participants indicated a high level of satisfaction with the system.
“The combination of safety outcomes and high satisfaction scores seen in this population, who were able to continue these existing medications during the study, shows that Control-IQ technology could be a powerful management solution for their therapy needs,” Jordan Pinsker, vice president and medical director for Tandem, said in a statement.