Dive Brief:
- Tandem Diabetes Care has received 510(k) clearance for its Mobi insulin pump, a smaller durable pump that can be controlled from a smartphone.
- The company plans a limited release of the device later this year, and a full launch in early 2024.
- The device is the first form-factor change in Tandem’s history, and could “meaningfully reaccelerate growth in 2024,” Craig-Hallum analyst Alexander Nowak wrote in a research note on Tuesday.
Dive Insight:
As competitors shift to smaller form factors, such as Insulet’s pump patches, Tandem has responded with its own smaller version of a durable pump.
Nowak wrote that the pump provides a new option for people with diabetes who currently take insulin through multiple daily injections (MDI), “especially to those who want a small discrete device, but not a permanently attached patch pump.”
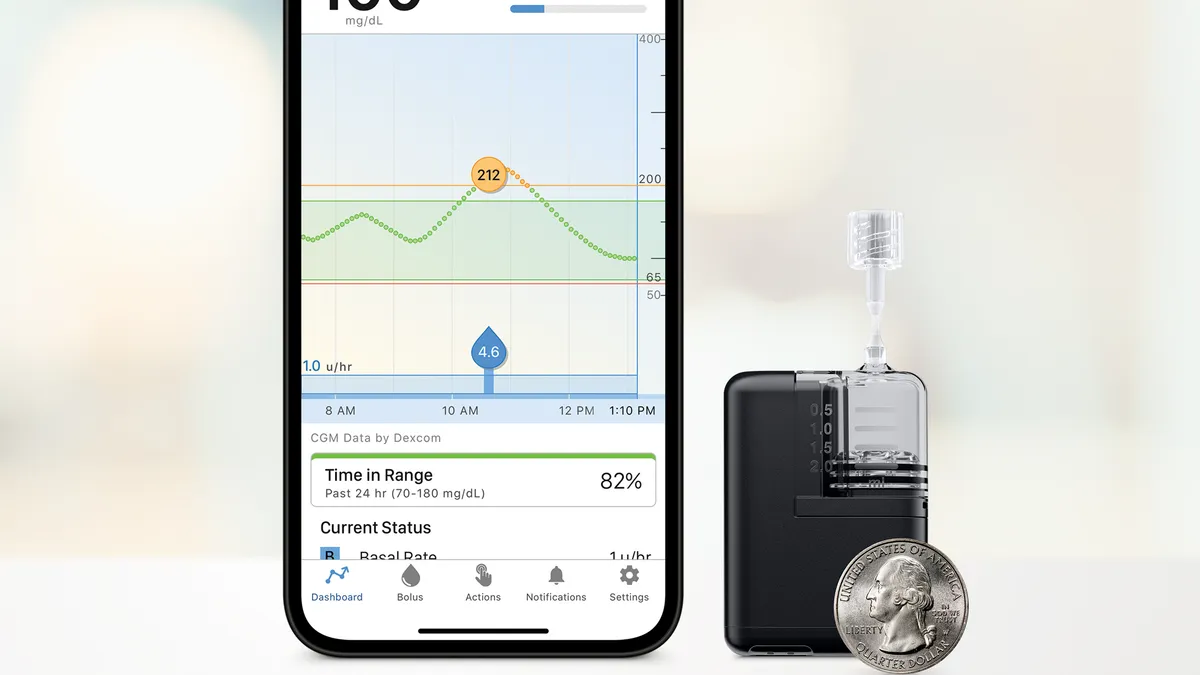
Mobi is about half the size of Tandem’s t:slim X2 pump, which Tandem introduced in 2018. The company claims it is the world’s smallest durable insulin pump.
The device can be clipped to clothing or worn on the body using a separate adhesive sleeve, and it can be controlled using a smartphone. It can also work with detachable infusion sets, allowing people to temporarily disconnect from the pump.
The device is cleared for people ages 6 and up with diabetes. It is also compatible with Tandem’s control IQ hybrid-closed loop algorithm, which is cleared for people with Type 1 diabetes, and allows the pump to be paired with a continuous glucose monitor to adjust insulin every five minutes, although people can still manually enter mealtime insulin doses.
Tandem CEO John Sheridan said in a February earnings call that Mobi is intended to offer people more choice in how they want to wear and operate their pump.
“As we've discussed in the past, our research shows that Mobi largely appeals to a segment of people who otherwise would not adopt insulin pump therapy with the options available today and is a catalyst for driving further market growth,” Sheridan said.
Nowak wrote that he could see patients upgrade from the t:slim X2 to Mobi. He also expects integration with new continuous glucose monitors, such as Dexcom’s G7 and Abbott’s Libre 3, to drive interest. Mobi should cost 15% to 20% less to produce, and have better margins, he added.
The device will also serve as a foundation for future products, including a patch pump Hybrid called Mobi Tubeless, Nowak wrote. The device will use the original Mobi pump, with an on-body disposable cartridge system that has a tubeless infusion set.
“Users could switch to tubeless on the fly and back to tube based on their activity levels. The company is also investigating Mobi’s use-case in the pharmacy reimbursement channel,” Nowak added.
In 2022, Tandem reported revenues of $801.2 million, a 14% increase year over year.