UPDATE: Sept. 23, 2019: Insulet said Monday morning its already commercialized Omnipod Dash insulin pump system has gained 510(k) clearance as an alternate controller enabled, or ACE, infusion pump. The ACE classification was established with FDA's De Novo clearance of Tandem's t:slim X2 insulin pump in February.
The new clearance enables the company to market the product as an integrated insulin pump that can work as part of an interoperable automated insulin delivery system. Insulet expects to bring an interoperable system, dubbed Horizon, to market in the second half of 2020. The algorithm for Horizon has yet to be authorized by FDA.
Analysts at Cowen called the news "an important step in Insulet's long term product pipeline," but said the near-term impact is minimal, in a note to investors Monday.
Dive Brief:
-
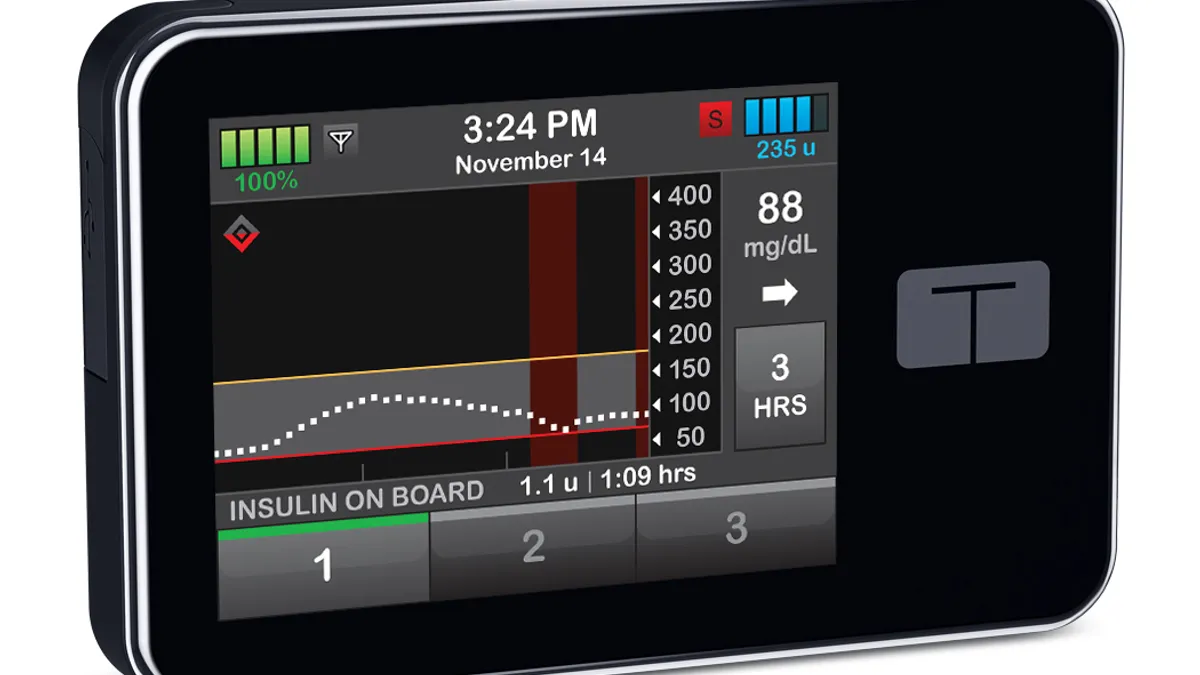
FDA has cleared the Tandem Diabetes Care t:slim X2 insulin pump for use under its De Novo premarket review pathway.
-
The device is the first insulin pump cleared by FDA that can work with an array of automated insulin dosing (AID) systems, continuous glucose monitors (CGMs) and other products used in diabetes management.
-
FDA thinks the interoperability of the insulin pump will enable diabetics to put together individualized diabetes therapy systems.
Dive Insight:
The clearance marks the first time FDA has signed off on such an interoperable insulin pump, leading the agency to talk up the significance of the regulatory action to patients.
“We’ve heard from the patient community that having the ability to customize their own diabetes management devices is important to them,” FDA commissioner Scott Gottlieb said in a statement. “The marketing authorization of the first [alternate controller enabled] insulin pump intended for interoperable use has the potential to aid patients who seek more individualized diabetes therapy systems.”
Tandem won the original FDA approval for t:slim X2 in June. At that stage, the pump, which holds 300 units of insulin and is connected to a touchscreen device, was integrated with the Dexcom G6 CGM.
The De Novo clearance now opens the door to use the pump with other brands of CGM and different types of electronic diabetes devices, such as blood glucose meters.
Gottlieb also envisages the De Novo clearance making it easier for developers of other connected diabetes devices to come to market. Tandem CEO Kim Blickenstaff touched on similar themes in his prepared remarks, stating that the action gives his company more flexibility to “make improvements to current products, create new products and collaborate with best-in-class companies in the development of future automated insulin delivery systems.”
The positioning of Tandem’s device at the heart of automated insulin delivery systems could support the anticipated growth in use of the technology. A survey commissioned by analysts at Leerink last year found physicians expect the number of Type 1 diabetics under their care who use t:slim to rise by 71% between 2017 and 2019. The same survey predicted an 89% increase in use in Type 2.
Tandem’s rivals for the insulin pump market could ultimately benefit from the De Novo clearance, though. The clearance resulted in FDA creating a new classification for ACE infusion pumps. FDA also created a set of special controls for the device classification covering the accuracy, reliability, cybersecurity and clinical relevance of the pumps. Companies can use the classification and controls to bring other interoperable pumps to market via the 510(k) pathway.