Dive Brief:
- Stereotaxis has secured Europe’s CE mark for its latest robotic surgery system that is designed for easier installation, which the company hopes will boost sales.
- The new robot addresses hospital construction challenges that have impeded past sales and is meant to support commercial scalability, CEO David Fischel said Monday on an earnings call.
- The St. Louis-based company also submitted a 510(k) application to the Food and Drug Administration to bring the platform, called GenesisX, to the U.S. market.
Dive Insight:
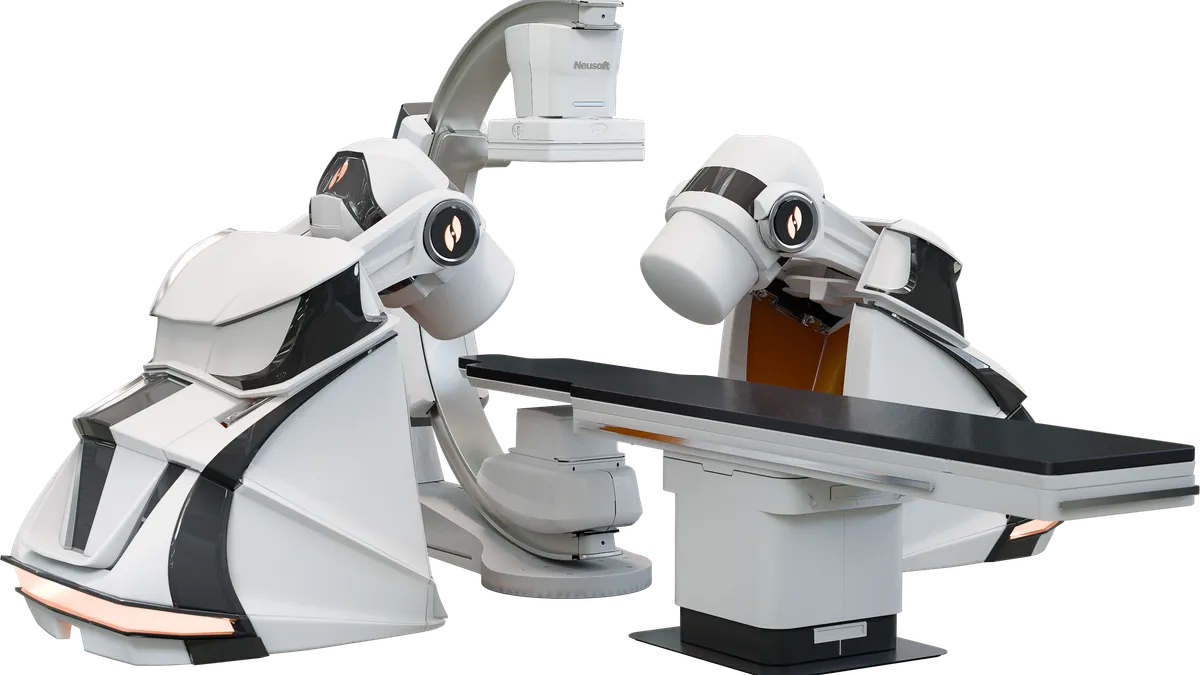
Stereotaxis’ robotic systems are used to perform minimally invasive endovascular procedures through magnetic navigation. Magnets on the robot’s arms generate a magnetic field controlled by the physician to direct the movement of the catheter.
GenesisX is the company’s third robotic platform, following the release of the Niobe system in 2003 and Genesis in 2020.
The new platform incorporates magnets that are significantly smaller than before, a robotic base with magnetic shielding built in, and more streamlined and sophisticated electronics throughout the system, the company said.
Stereotaxis hopes to translate physician interest in robotics into adoption and commercial growth with the new design, Fischel told analysts on the call.
The company posted second-quarter revenue of $4.5 million, down from $7.9 million a year ago.
“We have had hundreds of physicians express genuine interest in our technology since launching Genesis. Over 95% never end up getting the robot,” Fischel said. “The single largest impediment is the reliance on hospital construction and the long extended timeline that creates, along with the complexity of translating physician clinical interest into full organizational movement at the hospital.”
The company’s Niobe and Genesis systems require architectural planning and construction for installation that includes thousands of pounds of magnetic shielding in the walls, reinforcement of the floor and extensive electrical work, adding to hospital costs. The complexity of coordinating a project “leads many potential deals to stall or fizzle away,” Fischel said.
“In the fortunate cases where a robotic sale comes to fruition, we and the interested customers work through a multiyear sales cycle before translating interest into actual use,” the CEO said.
Although its technology has established clinical benefits and is “well received” by the physicians who use the robots, Stereotaxis holds less than 1% share in a “huge and highly attractive market,” Fischel said.
The GenesisX system uses smaller magnets that are stored in magnetic shielding built into the robot’s base, eliminating the need for shielding in the operating room walls. The platform also requires no structural anchoring through the floor and uses standard power outlets.
“GenesisX allows us to transition from a construction model to a placement model,” Fischel said.
FDA clearance for the system is expected by year end. Stereotaxis said it is also advancing toward regulatory approval in Europe and the U.S. for a compatible ablation catheter called Magic.
The company said its commercial results were below expectations in the first half of the year, but it is confident that a capital backlog will result in improved revenue and cash flow in the second half, and additional orders are anticipated near-term.
Stereotaxis expects to end the year with about $13 million in cash and no debt, enabling it to fund commercialization and reach profitability without additional financing.
In July, the company closed the acquisition of Access Point Technologies, a developer of electrophysiology catheters.