Dive Brief:
- Privately held Accelus, which makes a robotic navigation platform for spinal surgery, said it received FDA 510(k) clearance for use of the technology with GE Healthcare’s fluoroscopic imaging systems.
- The clearance allows the robotic system to be used in smaller hospitals and ambulatory surgery centers, increasing its attractiveness to spine surgeons, Accelus Chief Executive Officer Chris Walsh said in a statement.
- Called Remi, the device already has FDA clearance for use with a Medtronic 3D imaging system, which it gained in February 2021, and with 3D systems from GE and Ziehm as well as a CT imaging system from Stryker, which it received in October 2022.
Dive Insight:
High capital costs for both robotic and 3D imaging systems have been a barrier to adoption of robotic and navigated technology for spine surgery, Accelus said. The need for a large operating room footprint and issues with procedural workflow disruptions have posed additional challenges.
When Zimmer Biomet spun off its spine and dental businesses last year to form ZimVie, executives at the new company said Zimmer’s Rosa robotic system would not be a key part of its growth plans in the $12 billion spine market. ZimVie CEO Vafa Jamali cited low adoption rates for robotics in spine procedures as a deterrent.
Medtronic has been the market leader in robot-assisted spine surgery with its Mazor X system, which it purchased from Israeli company Mazor Robotics in 2018. Globus Medical, which last month agreed to buy Nuvasive in a $3.1 billion deal, also has a robotic navigation platform, called ExcelsiusGPS.
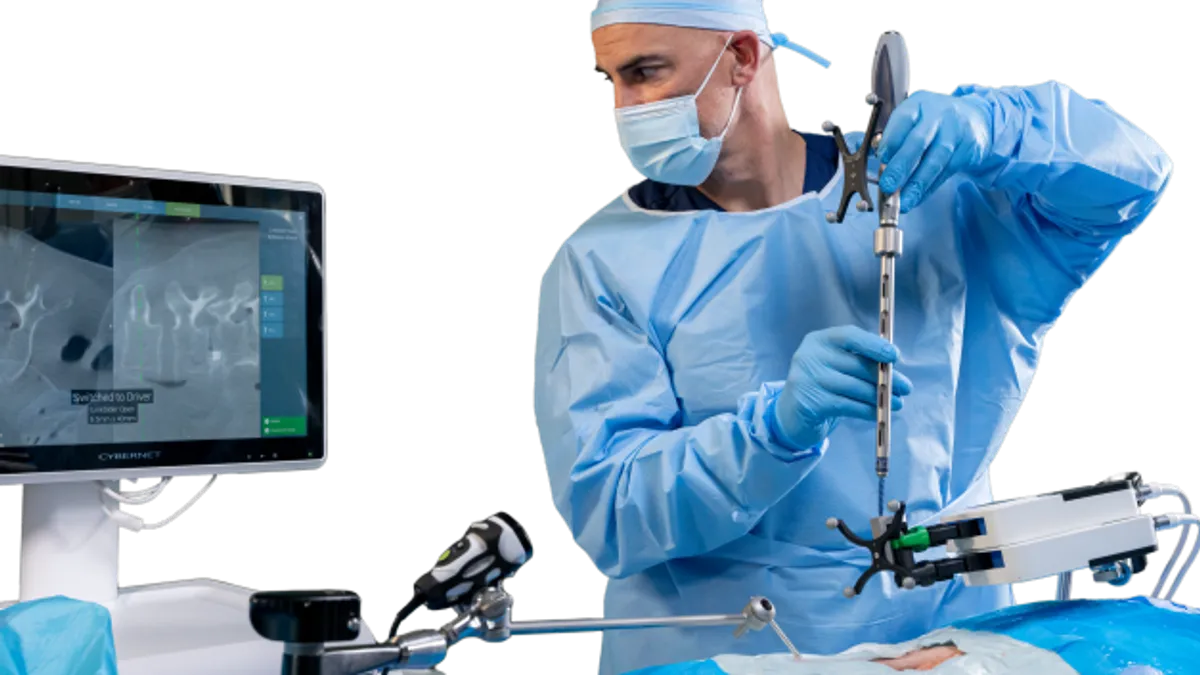
The Remi robot aims to address issues such as high cost and space constraints, Accelus said. The system is a targeting and navigation platform that helps surgeons with pedicle screw placement in the lumbar spine in minimally invasive procedures. Its camera tracks spinal instruments relative to an anatomical model based on a 3D scan or 2D fluoroscopic images of the patient.
A small and portable footprint allows the system to be used in various rooms and for multiple procedures in a day, and its simplified workflow is designed to reduce the learning curve for surgeons, the Palm Beach Gardens, Fla.-based company said.
“Most hospitals and ambulatory surgery centers (ASCs) are already utilizing C-arm X-ray fluoroscopy in their surgeries, which means they do not need additional imaging technology to utilize the Remi Robotic Navigation System, thanks to Remi’s most current FDA clearance,” Walsh said.
Initial surgeries with the Remi 2D are expected to begin in July, and a full commercial launch of the system is anticipated late in the third quarter, Accelus said.
Accelus was established in 2021 through the combination of Integrity Implants and Fusion Robotics.