Dive Brief:
- Smiths Medical recalled tracheostomy kits because of a manufacturing defect that could cause the balloon to separate from the inflation line.
- The recall, which includes the Portex Bluselect, Blugriggs and Bluperc kits, comprises more than 850,000 units, according to Food and Drug Administration database entries posted Wednesday. In a June letter to customers, Smiths said it received more than 10 reports of serious injuries related to the problem.
- So far this year, Smiths has had seven Class I recalls, a designation the FDA uses for recalls with a risk of potential serious injury or death.
Dive Insight:
ICU Medical acquired Smiths in 2022 for $2.35 billion. Smiths, which makes infusion pumps and airway management devices, has faced quality problems, including a warning letter in 2021 and numerous recalls over the past three years.
ICU Medical CEO Vivek Jain recently told investors the acquisition has been “more challenging than we had expected” amid the ongoing safety and quality issues.
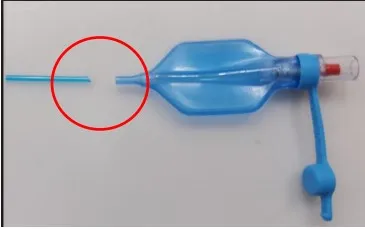
The latest recall involves tracheostomy tubes, which are used to support breathing when a patient’s airway is blocked. The pilot balloon used to inflate the tracheostomy cuff may become detached from the inflation line, causing the cuff to lose pressure. This can result in inadequate ventilation and increased risk of aspiration to the patient, Smiths wrote in its letter. Smiths instructed customers to discard all affected products and contact a representative for a replacement device or credit.
The company recalled a different line of tracheostomy tubes, called Bivona, in May because of a manufacturing defect. The recall was tied to one death and 35 injuries.
The FDA posted a second Smiths recall on Wednesday related to a problem with certain ParaPac ventilator kits. A knob that sets the tidal volume, or the amount of air delivered with each breath, can potentially move from the original setting when set at high and low levels. The problem affects more than 12,000 devices.














