Dive Brief:
- Siemens Healthineers received premarket approval for the 3D portion of its first completely redesigned mammography platform in over a decade, the company said Monday.
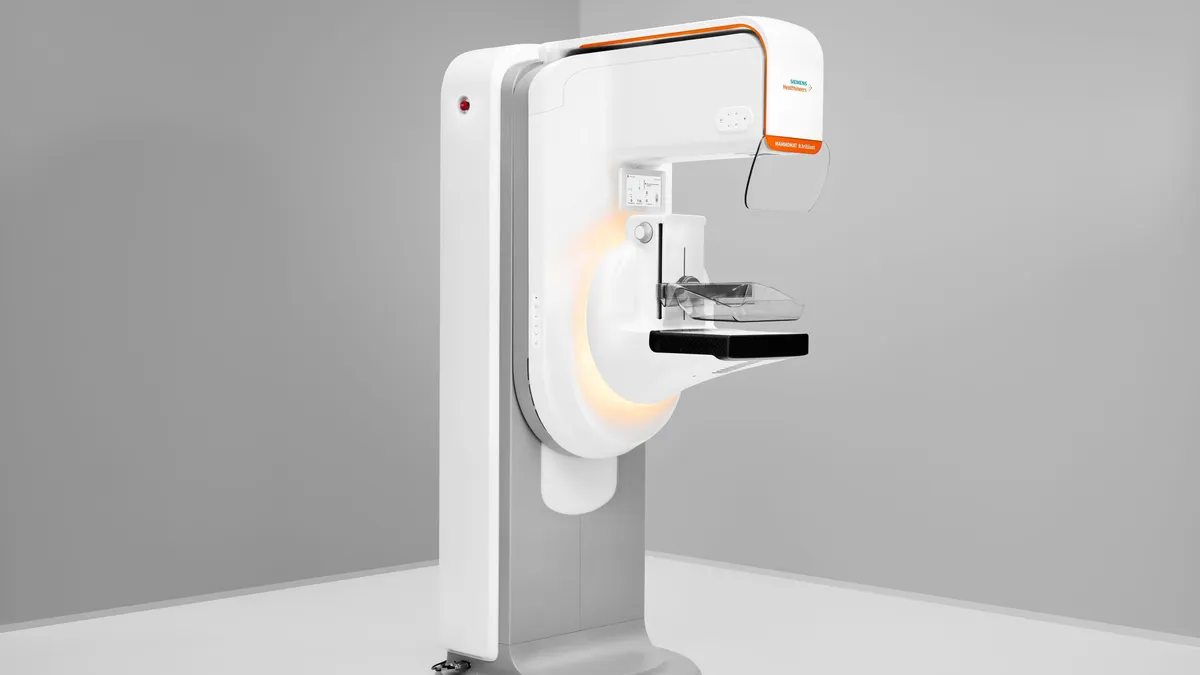
- The platform, Mammomat B.brilliant, features new 3D image acquisition and reconstruction technology. Using wide-angle technology, Siemens said it has overcome the issue of overlapping breast tissue which is a challenge with 2D imaging. The 3D technology can help visualize lesions that would have been obscured with the use of other imaging systems.
- Siemens has designed the system to identify abnormalities and microcalcifications in the breast with a high level of accuracy and to accelerate image acquisition.
Dive Insight:
Siemens received 510(k) clearance for its Mammomat B.brilliant mammography system in April. The Food and Drug Administration clearance covered elements involving 2D breast imaging, breast biopsy and titanium contrast-enhanced mammography. Separately, Siemens filed for premarket approval of the 3D portion of the system.
The FDA has now approved the 3D elements, which feature a 50-degree wide-angle technology. Siemens said the angle is the widest available.
The mammography system has a scan time of around five seconds, about 35% faster than other devices, Siemens Healthineers CEO Bernd Montag said in a May earnings call.
“It provides the fastest wide-angle tomosynthesis on the market, creating a 3D image with highest depth resolution in the shortest time possible,” Montag said. “In this way, abnormalities and microcalcifications in the tissue of the breast can be identified with a high level of accuracy.”
Tomosynthesis refers to the process of using X-rays to obtain sectional images of the breast and then reconstructing the images into a 3D mammogram. GE Healthcare, Hologic and Fujifilm also sell breast tomosynthesis systems in the U.S.
Compared to its predecessor, Mammomat Revelation, Siemens said the new system has improvements to patient comfort, user workflow and ergonomics. The patient can lean into the system during image acquisition for greater stability.
The approval follows FDA updates to the information on a final rule to amend the Mammography Quality Standards Act (MQSA). Under MQSA, mammography facilities must be certified, accredited and undergo periodic reviews of their clinical images. Enforcement of the final rule began on Sept. 10, meaning all sites must now comply with applicable requirements including the breast density notification.
In 2023, the FDA updated the regulations to require facilities to notify patients about the density of their breasts. Dense tissue makes it harder to detect cancer using a mammogram and increases the risk of developing breast cancer.