Dive Brief:
- Shiratronics has raised $66 million to fund a pivotal trial of an implantable neuromodulation treatment for chronic migraine, the company said Wednesday.
- The trial is designed to evaluate the safety and effectiveness of the system in patients with treatment-resistant chronic migraine who have failed current medical therapies.
- The Series B funding will also help support the premarket approval process for the device and the initial commercial launch.
Dive Insight:
Researchers have studied occipital nerve stimulation for decades and generated clinical evidence of efficacy against migraine when simultaneously targeting the supraorbital nerve. Problems related to electrode and lead migration were challenges in studies of older systems.
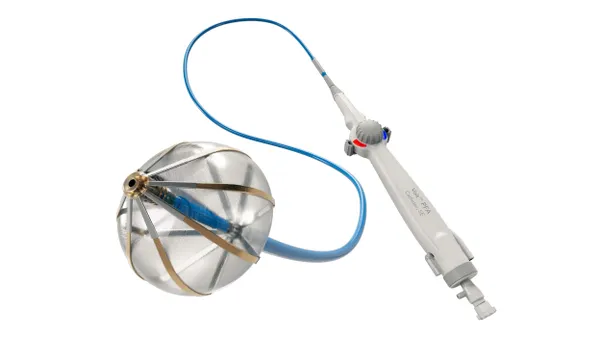
Shiratonics device is implanted beneath the skin in the head and delivers small electrical pulses to targeted nerves associated with migraine pain. The company tested the system at four sites in Australia. As of an update in June, all the adverse events, none of which were serious, had resolved quickly and spontaneously, Shiratronics said.
Shiratronics reported reductions in headache and migraine days in recipients of its breakthrough designated device. Mean migraine days fell from 18.9 to 6.8 after 12 weeks in the small study, which included 11 patients in the baseline analysis and 10 at the follow-up assessment.
Shiratronics’ next step is to validate the neuromodulation system in a Food and Drug Administration-approved, sham-controlled pivotal trial. Investigators are enrolling people who have 15 or more headache days a month despite taking medical therapies. Some patients in the Australian study had tried drugs including Botox and anti-CGRP treatments, a relatively new class of medicine, but were still having migraines.
New investor Norwest Venture Partners led the funding round, with the support of other first-time backers including Seroba and returning financiers such as U.S. Venture Partners.