Dive Brief:
- Senseonics has received an integrated continuous glucose monitoring (iCGM) designation from the Food and Drug Administration, the company said Tuesday.
- The designation, which the FDA granted via its de novo pathway, clears Senseonics to integrate its implantable CGM with pumps as part of automated insulin delivery systems.
- Senseonics’ sales are small compared to the CGM leaders Abbott and Dexcom, but BTIG analysts said the iCGM designation and planned one-year sensor could help the device appeal to a broader patient population.
Dive Insight:
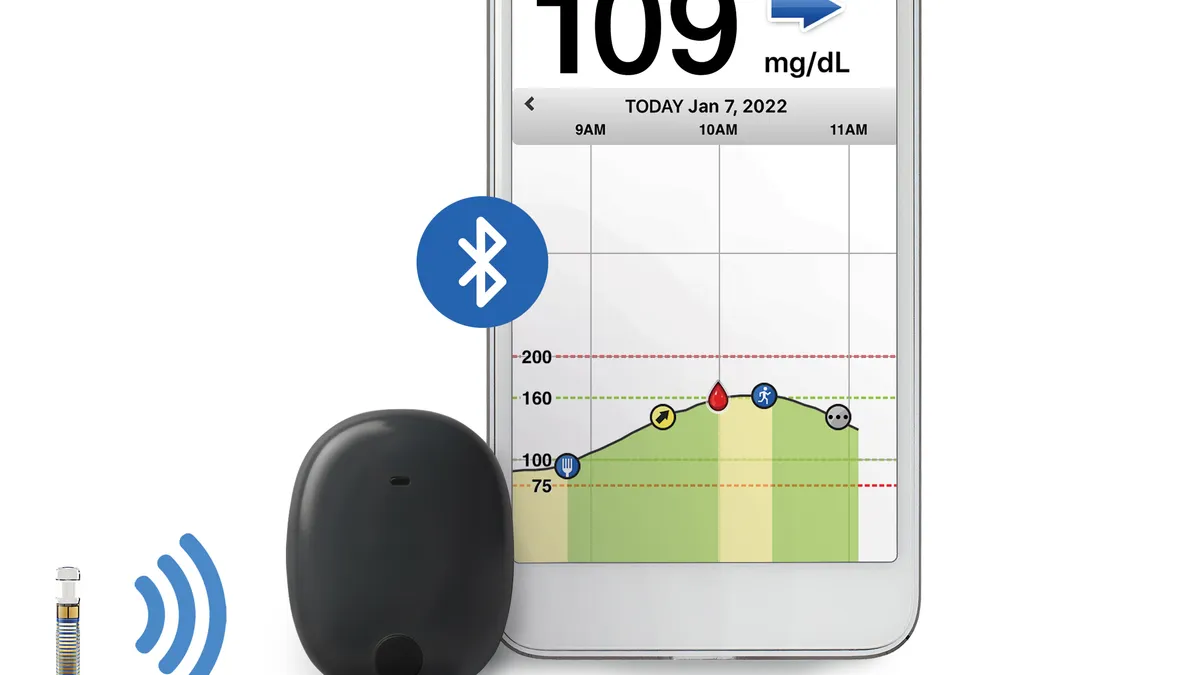
Senseonics’ Eversense device is a CGM implant that replaces fingerstick blood glucose measurements. The current device continually measures glucose levels for up to 180 days but requires daily calibrating with a fingerstick after day 21 and when symptoms do not match CGM information. Abbott and Dexcom sell shorter-lasting patches that dominate the CGM space.
The iCGM designation allows Eversense to integrate with compatible medical devices such as insulin pumps. Senseonics plans to advance talks with pump manufacturers about integrations that would use its implant as part of systems that automatically adjust insulin delivery based on CGM data.
Abbott, Dexcom and Medtronic already provide CGMs for use in automated systems, but their devices are shorter-lasting patches. Senseonics’ business is built on the idea that there is a market for implants that free patients from patches that need replacing around every two weeks. Last year, Senseonics reported revenues of $22.4 million, compared to $3.62 billion at Dexcom.
On an earnings call in February, Senseonics CEO Tim Goodnow said iCGM designation would provide a new option for patients who inject insulin but are considering pumps, as well as “an avenue to get into the 1 million or so patients that currently are linked together and doing sensor-augmented pumping.”
BTIG analysts wrote in a note to investors that the iCGM designation, alongside a longer-duration sensor, “will make Eversense more appealing to the larger segment of the population due to its broader integration potential. However, we have yet to see consistent adoption of Eversense.”
The analysts said the update is positive for Senseonics’ pipeline, “as the company planned on submitting the application for the 365-day version following approval of the iCGM designation for Eversense.”
Goodnow set out the sequence of events on the earnings call, telling investors that Senseonics is aiming for approval of its 365-day sensor in the fourth quarter. Senseonics can now get iCGM status via the 510(k) pathway by complying with special controls the FDA created through the de novo authorization.
Senseonics sees the longer-lasting implant as the basis for Gemini and Freedom self-powered versions of its implants that free patients from the need to wear an on-body transmitter. First-in-human testing of Gemini is scheduled to start in the second half of 2024.