Dive Brief:
- Roche shared details of its plans to enter the continuous glucose monitor (CGM) market, unveiling the Accu-Chek Smartguide at the Advanced Technologies and Treatments for Diabetes conference in Florence, Italy.
- On Thursday, the company released data on a 14-day sensor that could compete with Abbott and Dexcom for the CGM market. Roche is working to get a CE mark and plans to sell the device in “selected European countries” soon after receiving clearance.
- BTIG and J.P. Morgan analysts do not see Roche’s CGM as a major threat to Abbott and Dexcom, in part because, unlike the incumbents, the new device requires a finger stick calibration.
Dive Insight:
Roche is arriving late to a market dominated by two big, well-established rivals. Faced with the challenge of winning market share, Roche has identified glucose prediction as a way to differentiate its CGM from the competition in Type 1 and Type 2 diabetes.
“It's a novel CGM solution to help acting proactively. That is the key of our message,” Jochen Berchtold, Roche’s franchise lead for insulin therapy solutions, said at a media event to present the device. “It's more than just one sensor that generates the data. It's a combination of digital assets with AI-based predictive algorithms that really support people.”
The CGM pairs with two apps: one that displays current glucose data and another that hosts the three main algorithms that are central to Roche’s pitch for the market. One algorithm predicts if the user is at risk of hypoglycemia when they go to sleep, a feature Roche sees as a way to address the nighttime anxiety that affects some people with diabetes.
Another algorithm predicts how glucose levels will change over the next two hours. That sets the device apart from Abbott and Dexcom’s apps, which show trend arrows that predict how glucose levels will change in the next 15 and 30 minutes, respectively.
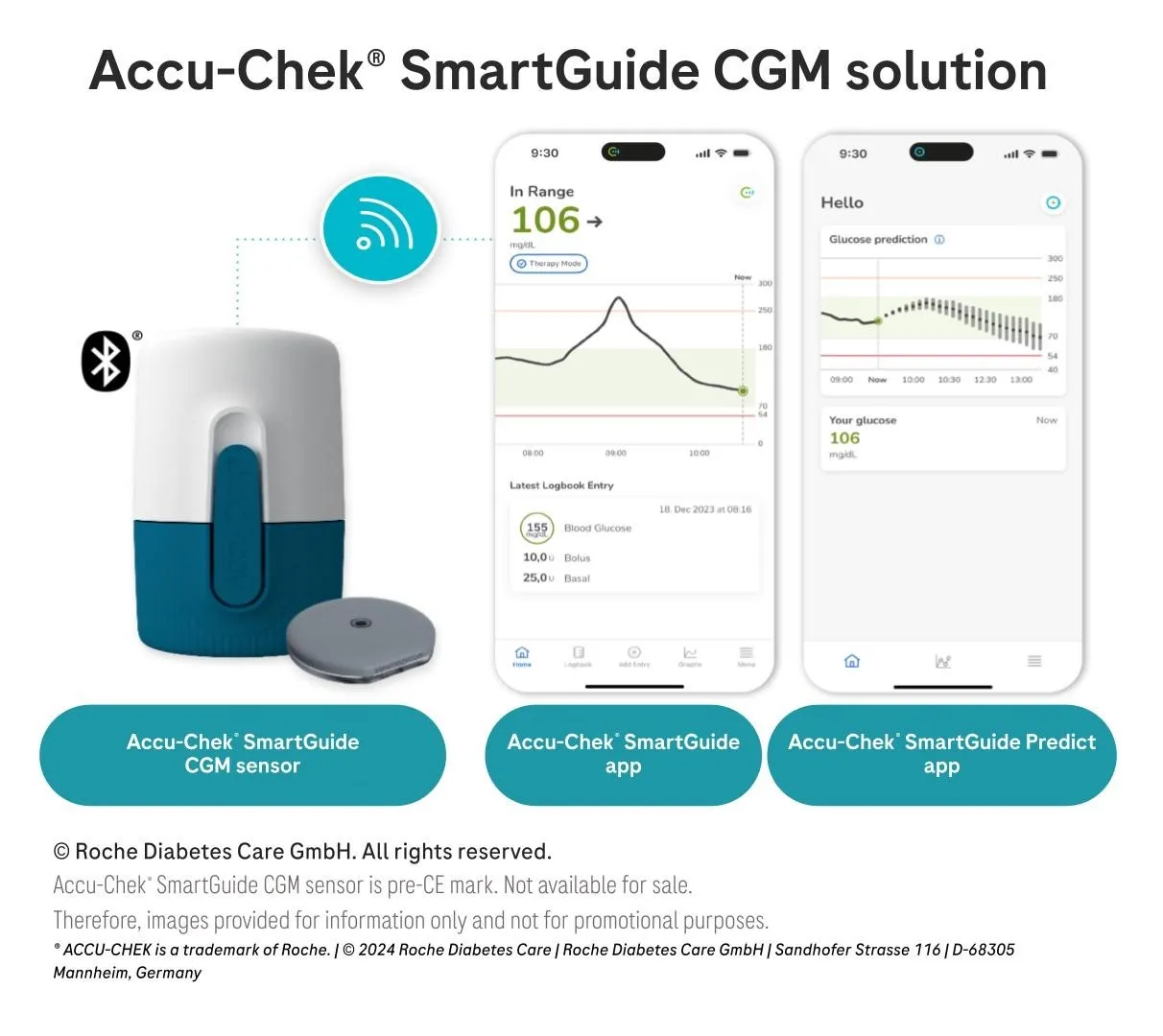
Roche’s app displays a two-hour prediction with error bars to show the range of potential future glucose values. The Swiss company has generated evidence that extending glucose prediction to two hours could reduce hypoglycemia distress and fear of hypoglycemia in people with Type 1 and 2 diabetes.
The algorithm can make predictions without users inputting information such as their consumption of carbohydrates and insulin dose. The algorithm performs better if users provide that information, but it is “not a massive improvement,” Berchtold said.
In a note to investors, BTIG analysts called the longer predictive algorithm a “nice feature that can help reduce stress for patients.” However, the analysts “are not sure how accurate this algorithm is or if most real-world users will find it a meaningful differentiator.”
The analysts identified the need to calibrate the CGM device with a finger stick initially as a “drawback” compared to Abbott’s and Dexcom’s products. J.P. Morgan analysts went further, telling investors, “Global markets have shifted entirely away from finger sticks, and we think walking backward regardless of form factor is a complete non-starter.”
Performance data added to the skepticism of the J.P. Morgan analysts. Roche reported an overall mean absolute relative difference (MARD) of 9.2% with finger sticks. Dexcom G7 and Abbott’s Freestyle Libre 3 have MARDs of 8.2% and 7.8%, respectively, without finger stick calibration.
The device has a “sleeker form factor” than the J.P. Morgan analysts expected. Images shown during the media event showed a similar design to existing CGMs, although Roche is yet to share the exact footprint of its device. Accu-Chek Smartguide’s 14-day lifespan matches Abbott’s Freestyle Libre 3 and beats G7, which lasts 10 days. Dexcom’s new over-the-counter CGM has a 15-day lifespan.
Roche has yet to discuss the timing of a CE mark or its pricing plans.
J.P. Morgan analysts see little scope to undercut Abbott and Dexcom “given their scale and volumes.” Roche has also yet to share the timeline for integrating the CGM with its insulin pumps, although Berchtold confirmed the work is part of the development strategy but not part of “the launch sequence.”












