Between rising insulin pump use, an aggressive shift to automated manufacturing and Medicare initiating coverage, adoption of continuous glucose monitors was poised to truly take off in 2019. The year delivered.
CGMs are devices that track blood sugar using a below-the-skin sensor. A wirelessly connected transmitter can stream that information to an external reader device.
For individuals seeking ongoing tracking of blood glucose levels to better understand their condition and avoid dangerous highs and lows, the technology can replace finger pricks, test strips and blood glucose meters. The volume of devices sold in the U.S. has grown by about 60% in 2019, according to Ryan Blicker, a vice president of healthcare equity research at Cowen, with adoption driven at the most basic level by improving ease of use and affordability.
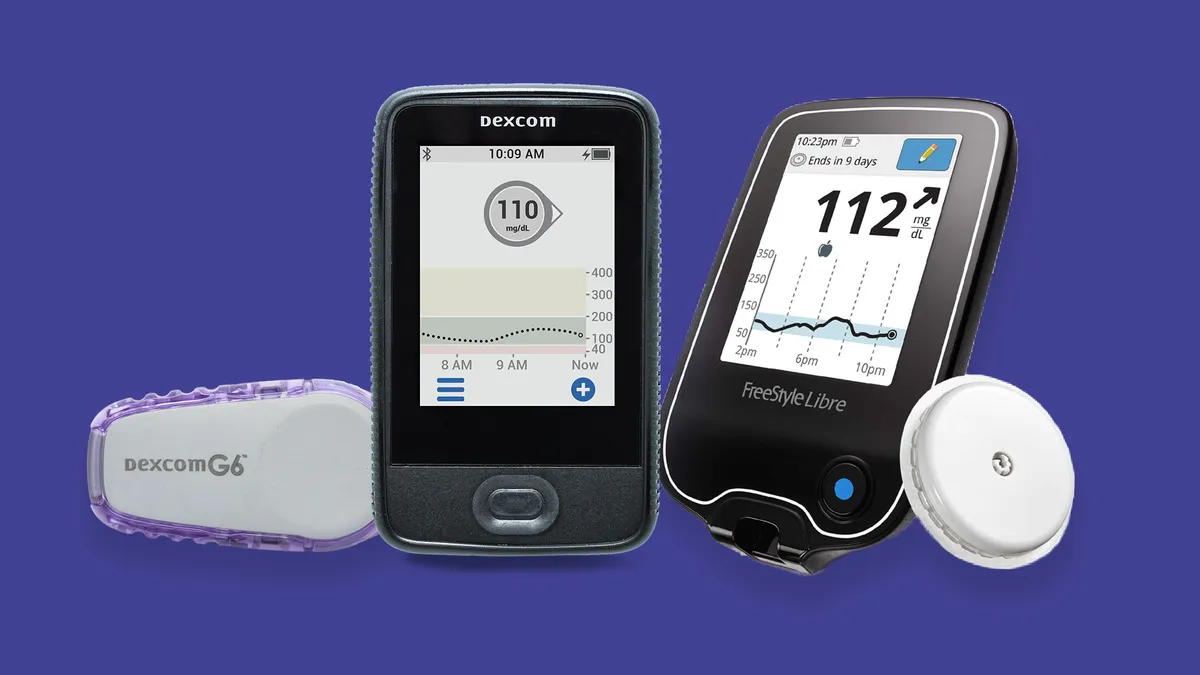
Both medtech giant Abbott and smaller rival Dexcom, which has specialized in diabetes tech since its founding in 1999, market FDA-approved CGMs requiring minimal calibration. Dexcom's latest device, the G6, was launched in 2018 with a sensor lasting 10 days, while Abbott's FreeStyle Libre 10-day device was approved in 2017, with a 14-day label greenlighted about a year later.
Abbott and Dexcom aren't alone in recognizing the opportunity in CGM; incumbent Medtronic and startup Senseonics are among the medtechs, large and small, also seeking a slice of the market. But Abbott and Dexcom are "clearly the two lead horses," Blicker said. "I think those two are going to be a duopoly here over the next five years."
The market appears to be plenty big, with about 1.25 million Americans in the Type 1 population.
Dexcom saw nearly 45% year-over-year revenue growth to approximately $617 million in the first half of 2019, and Abbott Diabetes' notched a 31% uptick to $1.17 billion in the same comparable period.

An additional 27 million with Type 2 and an estimated more than 84 million with prediabetes provide potential market expansion. Both Abbott and Dexcom have noted the technology's potential to measure other analytes or be used by people without diabetes who simply want to more closely track how different nutrition choices affect their body, for instance.
The rivals have pledged to pursue all sections of the diabetes market, but both have carved out specialties. Dexcom is widely considered to have more convenient design and premium technology for children with Type 1, for example, who are at greater risk of having a potentially deadly hypoglycemic episode while asleep and therefore benefit from the G6's alarm features.
The more affordable FreeStyle Libre system (Baird senior research analyst Jeff Johnson estimates its average cost per day is about $4, compared to G6's $7 or $8), on the other hand, lacks some of those alerts, and is a more attractive choice, perhaps, for adults looking to better manage their Type 2 diabetes. Abbott's lower costs have eroded Dexcom's prices, but rapidly growing adoption in an underpenetrated market make up for it.
"I think even Abbott has probably been surprised at how strong their Libre business has been," Johnson said.
"A few years ago, I'm sure sitting around the capital allocation table, Libre wasn't the star of the show. But over the last couple years, it's become obvious what a huge product they have on their hands and you've seen them now finally start to commit ... to much more significant allocations toward manufacturing capacity, things like that."
Now, it's a scaling game.
Abbott is investing in "ongoing manufacturing expansion of three to five times over the next several years," Bob Kunkler, vice president of global commercial operations at Abbott Diabetes Care, confirmed in an email.
Likewise, Dexcom plans to quadruple its G6 production capacity by next summer.
"When it comes to building thousands, and tens of thousands, and hundreds of thousands, the challenges of these manufacturing materials and these processes becomes very real," CEO Kevin Sayer told MedTech Dive in an interview. "We've been pushed to our limits on capacity the entire year because our growth has been so dramatic."
That adoption has been helped along by payer recognition of the benefits of CGM. Medicare began covering the devices for Type 1 patients in 2018; the next big reimbursement target is the insulin-dependent Type 2 population.
As uptake booms, the next versions of these devices are just around the corner. FDA approval of Abbott's FreeStyle Libre 2 has taken longer than Abbott expected but is considered imminent by analysts. Regardless, analysts don't expect the next-gen device to meaningfully hurt Dexcom's prospects, particularly as Dexcom plans to begin releasing the smaller G7, co-developed with Google's Verily, in late 2020.


 Read more
Read more







