Dive Brief:
- Recor Medical received approval from the Food and Drug Administration for its Paradise ultrasound renal denervation system to treat hypertension, making it the first company to offer the treatment in the U.S.
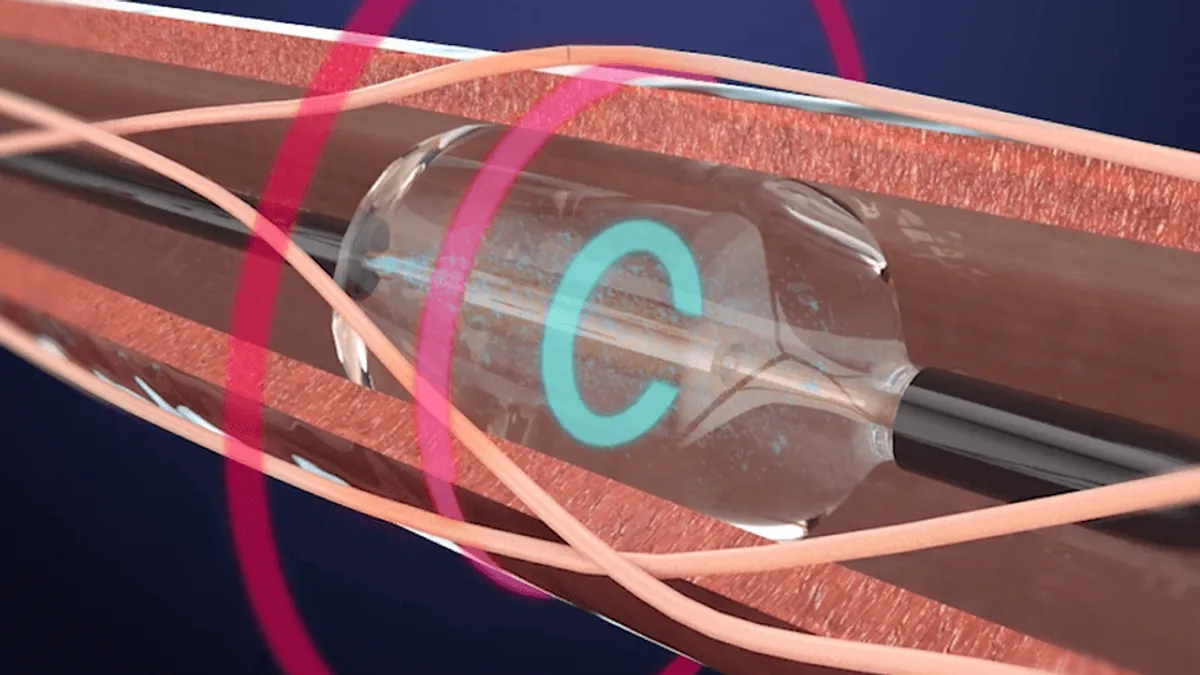
- Renal denervation is a minimally invasive procedure where energy is delivered through the renal arteries to ablate the surrounding nerves, with the idea that reducing activity in the renal nerves can lower blood pressure.
- An FDA panel in August recommended approval of Recor’s treatment, but did not recommend Medtronic’s competing device.
Dive Insight:
Recor, a subsidiary of Otsuka Medical Devices, and Medtronic are two competitors vying to bring their renal denervation treatments to the U.S.
Recor’s Paradise device received a CE mark in 2012 and is an investigational device in Japan. With the FDA’s approval, the device is indicated for patients where lifestyle changes and medications were not enough to control their blood pressure.
Recor’s device uses ultrasound energy to disrupt signals from sympathetic nerves, a slightly different mechanism than Medtronic’s Symplicity Spyral device, which uses radiofrequency energy. Medtronic received a CE mark for its device in 2013.
Outside advisers recommended that the FDA approve Recor’s system by a 10-2 vote in August but said more study is needed on the long-term impact of the procedure. They raised questions about which patients may benefit most and the durability of the treatment after the first two months.
In the same meeting, advisers voted 6-7 against approving Medtronic’s Symplicity Spyral system, noting that one trial of the device failed to meet its primary endpoint and the other trial showed limited clinical benefits.
The FDA appears to be unlikely to approve the device, given the advisory vote, Needham analyst Mike Matson wrote in a research note in August, although the agency could choose to go against the panel recommendation.