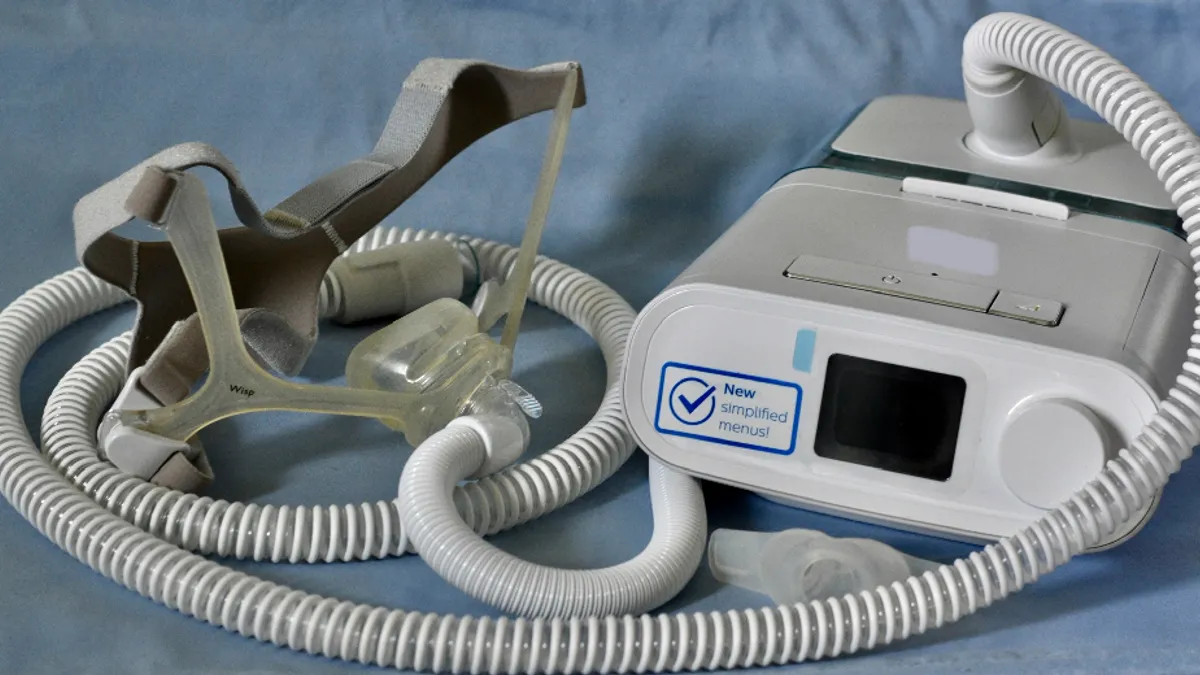
As Philips, the world’s fourth-largest medtech manufacturer by revenue, remains dogged by a recall of its sleep apnea respirators, U.S.-based competitor ResMed is poised to increase its share of the growing market for the devices used by millions of Americans, analysts said.
“ResMed remains well-positioned to gain sustainable market share and remain the market leader,” William Blair analysts led by Margaret Kaczor wrote in a note released today. ResMed is scheduled to release its third-quarter earnings on Thursday.
In a conference call this week discussing his company’s third-quarter earnings, Philips CEO Roy Jakobs said the recall had yet to be resolved. Some 5.5 million CPAP, BiPAP and other ventilators were recalled and Philips has been unable to ramp up production and source enough new parts to repair or replace the defective machines.
Last week, Jakobs apologized in a blog post for the company’s botched recall. Although Philips was aware for years of problems with the respirators, it only began a recall in 2021 after the CEO said the company’s senior management became aware of the severity of the issue.
The Food and Drug Administration said that through the end of July, it had received more than 69,000 medical device reports about issues with the Philips respirators, including 168 reports of deaths allegedly connected to the devices. Most of the complaints concern a sound-proofing foam that disintegrated and was sucked into users’ lungs through the device.
“Industry players we talked to said that they are starting to see supply improve, which could be a positive for ResMed in the coming quarters,” the William Blair analysts wrote. “There continues to be a lack of clarity as to when Philips will reenter the market, particularly with the looming FDA consent decree, suggesting to us that ResMed still has runway to gain market share as supply seems to be improving.”
While Philips says it plans to have largely completed the replacement or repair of recalled apnea devices by the first quarter of 2023, the company’s ongoing discussions with the U.S. Justice Department on a consent decree governing the respirators has left “little clarity” on when Philips will be back in the market selling devices, the analysts wrote.
“While it is unclear how long Philips will be out of the market, we believe that providers will want to source from multiple providers going forward,” including Philips, they said.
Given the demand for devices, “ResMed should be well-positioned to take market share during the recovery and leverage these share gains once the market returns to a normal state,” the analysts added.