Dive Brief:
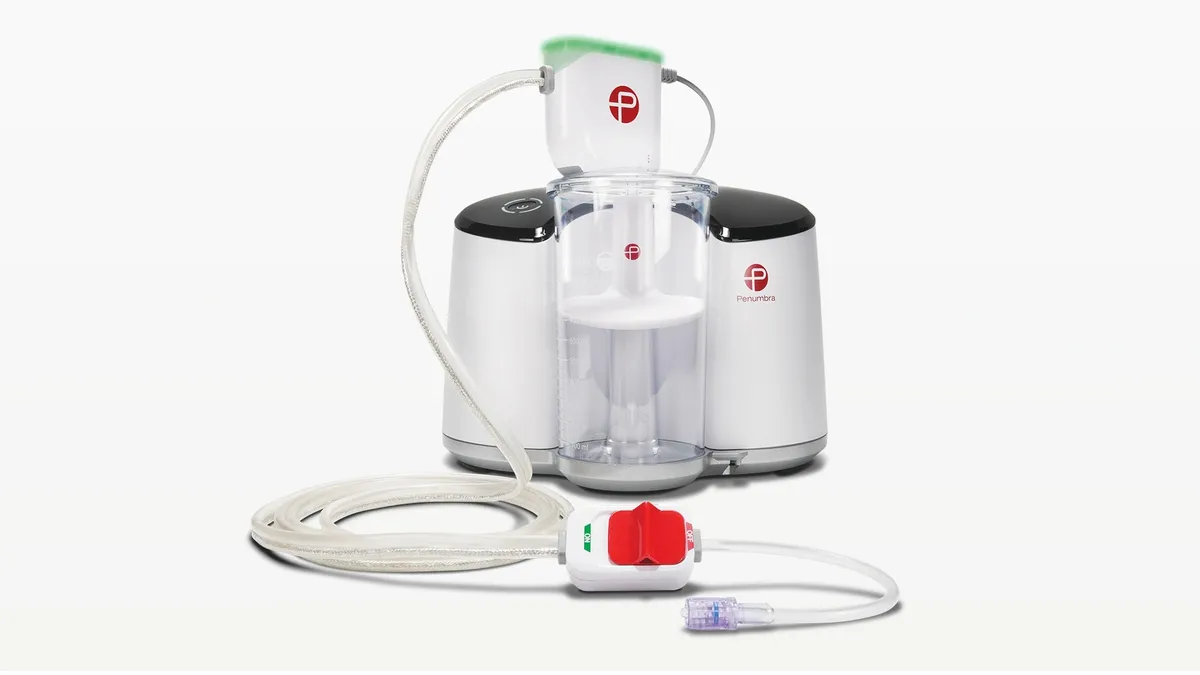
- Penumbra shared interim results supporting the safety and effectiveness of its computer-aided vacuum thrombectomy system for treating pulmonary embolism.
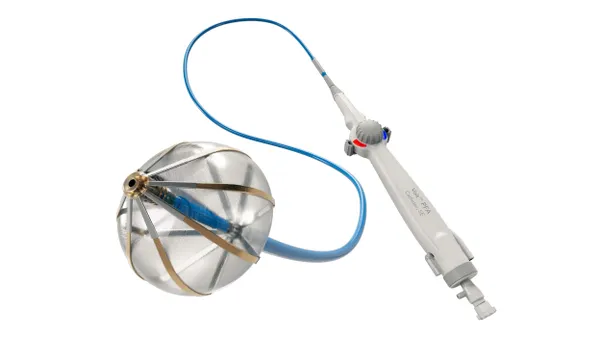
- The single-arm study showed a 25.7% reduction in the right ventricle/left ventricle ratio, a measure of right heart strain, using Penumbra’s Indigo aspiration system.
- The company has another trial planned, called STORM PE, comparing its Indigo system paired with anticoagulants to taking anticoagulant drugs alone, BTIG analyst Ryan Zimmerman wrote in a research note.
Dive Insight:
Penumbra first received approval for its Indigo system with its Lightning technology in 2020. Earlier this year, the company received clearance from the Food and Drug Administration for its newer Lightning Flash technology to remove large clots while minimizing blood loss.
An interim analysis of 150 patients treated with Penumbra’s Indigo system with 90-day follow-up was presented at the Vascular Interventional Advances Conference (VIVA) on Wednesday.
Penumbra’s STRIKE-PE study met both of its primary safety and efficacy endpoints, showing the device reduced right heart strain. After 48 hours, patients’ right ventricle/left ventricle ratio decreased from 1.39 at baseline to 1.01, a 25.7% decrease, according to slides reviewed by MedTech Dive.
Four of the 150 patients, or 2.7%, experienced major adverse events within 48 hours. Those included four reports of major bleeding, two reports of device-related clinical deterioration and one report of device-related pulmonary vascular injury, according to study slides.
The analysis also tracked several quality of life measures after 90 days. It found that 92% of patients had very slight or no shortness of breath, compared to just 13% of patients at baseline. Their functional ability, measured by 6-minute walk distance, increased by 120 meters from discharge to 90 days. And 86% of patients had no symptoms of heart failure after 90 days, compared to 58% at discharge.
“The complexity of treating pulmonary embolisms appropriately cannot be overstated. They are often complicated to diagnose and, in many cases, if not treated quickly, can be life-threatening,” Ido Weinberg, a vascular medicine physician at the Massachusetts General Hospital who presented the data, said in a statement from Penumbra. ”While surviving a pulmonary embolism is a victory, patients regaining their quality of life and ability to function without ongoing limitation is critical.”
Anticoagulant drugs alone are the current standard of care for pulmonary embolism, BTIG’s Zimmerman wrote. He listed the STRIKE PE study as one of four key trials for Penumbra. The study, enrolling 600 patients, is expected to be initially completed by the third quarter of 2024, he said.
Another study to watch is STORM PE, the first randomized trial comparing mechanical aspiration thrombectomy to anticoagulation alone, Zimmerman wrote. That study will enroll 100 patients at 20 sites, also measuring changes in right ventricle/left ventricle ratio as its primary endpoint.