Dive Brief:
- FDA has authorized a resorbable implant under the De Novo premarket review pathway that fills the gap between the torn ends of a patient’s anterior cruciate ligament (ACL), one of the most common knee injuries in the U.S.
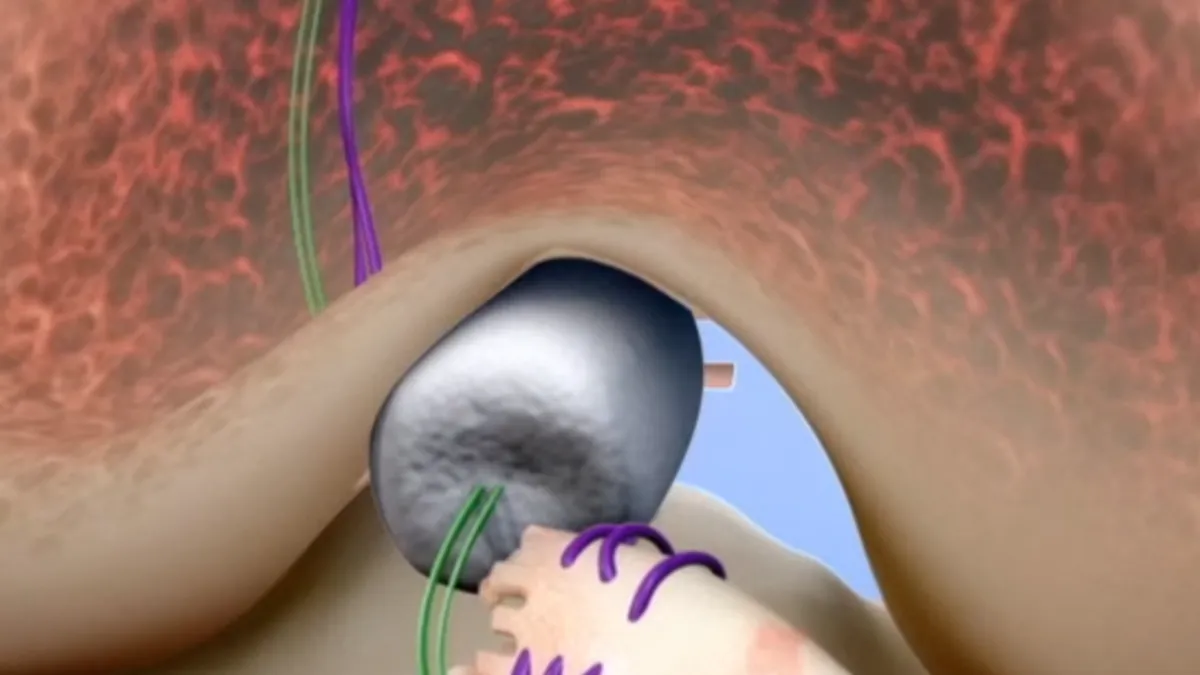
- Miach Orthopaedics' synthetic device, made from bovine collagen and absorbed by the body in about 8 weeks, offers an alternative to traditional and invasive ACL reconstruction used to treat tears with harvested tendons. The company's Bridge-Enhanced ACL Repair (BEAR) surgical implant is indicated for "skeletally mature" patients, ages 14 or older, with complete ACL ruptures confirmed by MRI.
- A surgeon secures the implant with sutures and injects the patient's own blood into it during the procedure "with the intent of forming a device-protected clot that enables the body’s healing process," FDA said. The agency deemed the device safe and effective based on a 100-patient randomized controlled trial.
Dive Insight:
Approximately 400,000 ACL injuries occur in the U.S. annually. However, for decades the most common orthopaedic treatment has been limited to invasive ACL reconstruction, which typically requires using tendons or a combination of tendons and bones harvested from patients' bodies or cadavers.
Miach's implant does not rely on harvested grafts and is the "only currently-available alternative to reconstruction with allograft, autograft or suture-only repair for the treatment of ACL rupture," according to FDA.
Patients must have an ACL stump attached to the tibia to construct the repair and the device must be implanted within 50 days of injury.
FDA assessed the safety and effectiveness of the implant in a randomized controlled trial of 100 patients with complete ACL rupture. In the trial, 65 subjects received the device and 35 underwent conventional autograft reconstruction using their own tendon from another part of the body. Both groups received physical therapy.
After the 2-year mark, FDA said outcomes were similar for pain, knee function, and sports activity based on the patient-reported International Knee Documentation Committee Subjective Score. Arthrometry evaluations at follow-up, used to measure the difference in laxity between a person’s healthy leg and their injured leg, showed nearly identical results for joint laxity.
Miach Orthopaedics, which is focused on bio-engineered technologies intended to facilitate new tissue growth, contends their implant is the first new treatment for ACL tears in more than 30 years.
The technology was pioneered by Martha Murray, professor of orthopaedic surgery at Boston Children’s Hospital, with initial research funding from NIH and the NFL Players Association before she founded the privately-held medtech located in Westborough, Massachusetts.
"ACL injuries are all too common among athletes of all ages and can be a career-ending injury in the NFL, which is why the NFL Players Association has been a sponsor of the BEAR clinical trials since the beginning,” said Sean Sansiveri, vice president of the association, in a written statement.
Miach Orthopaedics plans to conduct a limited market release of the BEAR implant in early 2021.