Dive Brief:
- Medtronic is recalling more than 45,000 cerebrospinal fluid (CSF) drainage and sampling tubes from the U.S. over an issue linked to 26 injuries.
- The Food and Drug Administration, which published details of the recall Thursday, explained that tubes can disconnect, leading to infections, leakage or excessive drainage of CSF, and abnormality of the ventricles.
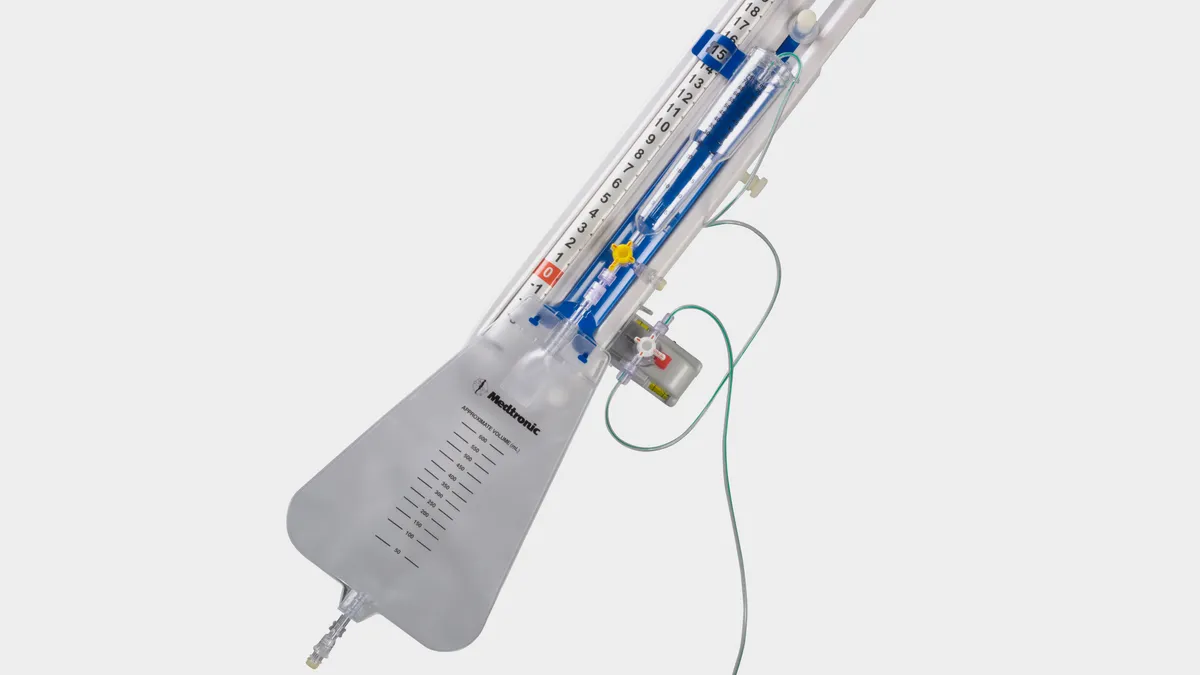
- Because uncontrolled over drainage of CSF could result in neurological injury or death, the FDA categorized the recall of the Duet External Drainage and Monitoring System as a Class I event.
Dive Insight:
The device is used for temporary drainage or sampling of CSF in people who are having open surgery for one of two types of aortic aneurysm, as well as in patients who develop symptoms such as paralysis of the legs after the procedure. CSF flows through a set of tubes that runs from an external lumbar catheter to a collection bag.
Medtronic began its recall because of the potential “for the catheter disconnection from the patient line stopcock connectors,” the FDA said. An investigation into the cause of the disconnections is underway.

The company sent an urgent medical device recall about the issue in January. In the notice, Medtronic asked customers to identify, quarantine and return all unused, non-expired products affected by the recall. Healthcare professionals should check all components that are attached to patients for damage and ensure connections are secure and leak-free, Medtronic said.
Medtronic advised healthcare professionals who find leaks or disconnections to switch to an alternative device using a sterile technique. The company recommended against removing or replacing Duet devices that are working as intended.
The recall has parallels to a letter Medtronic sent to users of the Duet system almost 10 years ago. In that earlier, Class I recall, the company warned that “the patient line tubing in the Duet External Drainage and Monitoring Systems may become disconnected from the patient line stopcock.”