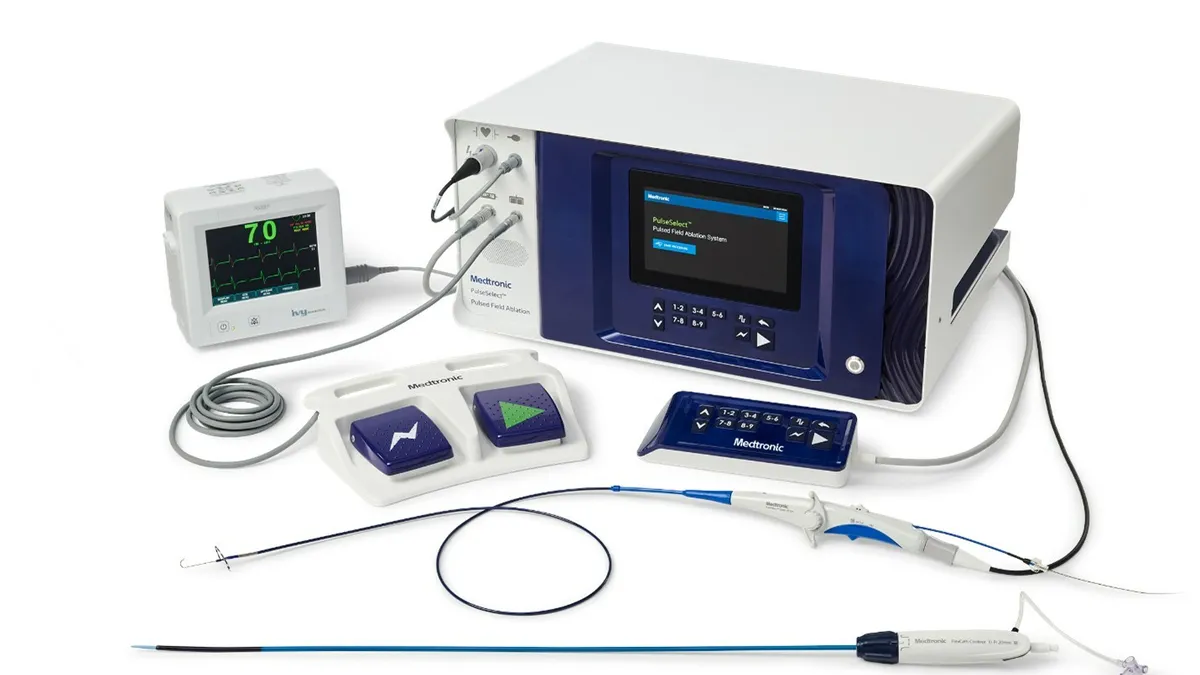
Dive Brief:
- Medtronic has gained the first approval from the Food and Drug Administration for a pulsed field ablation (PFA) system to treat atrial fibrillation (AFib), pulling ahead of other medtech companies in the race to bring the technology to the U.S. market.
- The treatment approach has garnered attention as a safer alternative to radiofrequency and cryoablation techniques for addressing the abnormal heart rhythm, and Boston Scientific and Johnson & Johnson are pursuing the market.
- Medtronic, in announcing the FDA’s approval for its PulseSelect PFA system, said the device has demonstrated a 0.7% safety event rate and clinical success rates of 80% in both paroxysmal and persistent AFib patients.
Dive Insight:
AFib, which can increase a patient’s risk of having a stroke, is expected to affect more than 12 million people in the U.S. by 2030. The number of cases is predicted to rise as the population ages.
Other cardiac ablation approaches to treat AFib use either heat (radiofrequency energy) or extreme cold (cryoablation) to create small scars to block AFib’s irregular heartbeats. Pulsed field ablation is a nonthermal method that uses short high-voltage pulses to target cardiac tissue.
“Because the mechanism of cell death is non-thermal, the risk of collateral structure damage is potentially lower,” Medtronic said.
Data showing the PFA technique offers a safer AFib ablation option has generated enthusiasm among electrophysiologists and predictions that the approach will be rapidly adopted.
Boston Scientific, which is preparing to bring its Farapulse PFA device to market in the U.S., has predicted a “dramatic shift” to the technology, with PFA expected to dominate the AFib treatment market in five years.
"The PulseSelect PFA system ushers the EP community to a new era of safe, effective, and efficient AF ablation that overcomes many challenges in our current practice," Amin Al-Ahmad, an electrophysiologist at St. David's Medical Center in Austin, Texas, who has used the device in Medtronic’s PULSED AF trial, said in a statement from Medtronic.
Medtronic said its PFA system, which received Europe’s CE mark last month, is designed to fit within the clinician’s preferred workflow.
“The learning curve in using the catheter and system is short, and the catheter enables the operator to deliver fast and controlled pulsed field energy for AF ablation," said Al-Ahmad.
Boston Scientific CEO Mike Mahoney said on an October earnings call that the company expects FDA approval of its Farapulse PFA system in the second half of 2024.