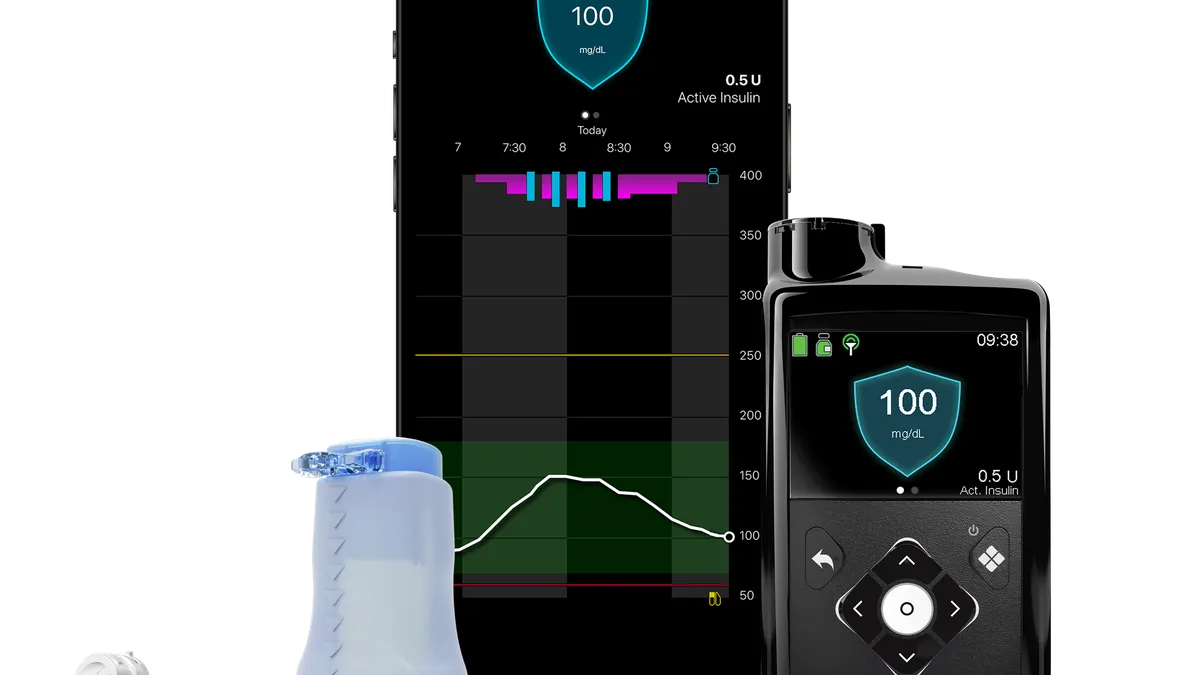
Dive Brief:
- Medtronic has used the American Diabetes Association (ADA) Scientific Sessions to show how its recently approved MiniMed 780G pump can improve the management of diabetes in children.
- At the event, Medtronic shared the results of a small clinical trial that found adolescent users of the pump can achieve improved blood glucose control without precisely counting carbohydrates.
- Another presentation covered real-world evidence on thousands of children and showed that the MiniMed 780G has the biggest effect on people with the poorest glycemic control at baseline.
Dive Insight:
Medtronic took longer than expected to bring its MiniMed 780G insulin pump to market in the U.S., with the regulatory problems at its diabetes unit delaying U.S. approval until April. Now, after seeing diabetes business sales fall in the last four years, the company is looking to the device to help it return to growth and win market share from rivals such as Insulet and Tandem Diabetes Care.
Against that backdrop, Medtronic arrived at the ADA on Friday with data showing the benefits of using the MiniMed 780G in children. The U.S. Food and Drug Administration approval covers the use of the device in people ages seven years and up.
In one trial, investigators randomized 34 people ages 12 to 18 years to use the insulin pump while either entering precise carbohydrate counts or using three fixed carbohydrate pre-sets. Time in range was lower in the pre-set group, at 73.5%, than in the precise cohort, at 80.3%, but the simplified approach still enabled the children to maintain international targets of glycemic control.
Goran Petrovski, the Sidra Medicine physician who presented the data, explained what a simpler model of carbohydrate counting could mean for patients in Medtronic’s statement about the ADA publications.
"Many individuals with Type 1 diabetes struggle with meal management, with nearly 50% considering carb counting the most burdensome aspect of diabetes management,” Petrovski said. “Indeed, many frequently underestimate their carbs or forget to bolus, and this has an adverse impact on clinical outcomes. This study shows that a simplified meal management approach with the MiniMed 780G system helped users maintain glycemic targets while providing forgiveness for inexact carb counts.”
In the other study, researchers analyzed real-world data on children in Europe. The analysis linked the use of the MiniMed 780G to improved time in range, with the biggest benefits seen in children with poor glycemic control at baseline, and reduced user-initiated boluses.