Dive Brief:
- Medtronic has identified a group of patients that may face a higher risk of problems with its HeartWare ventricular assist device (HVAD), the company said in an urgent medical device communication sent to customers last week.
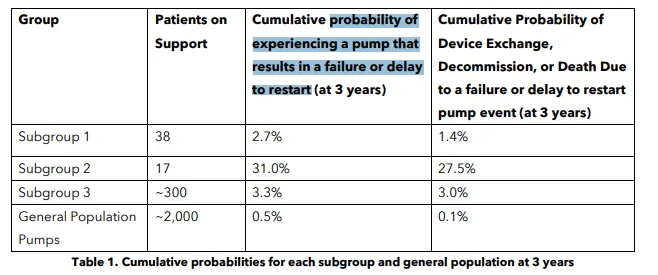
- The group of about 300 people has a 3.3% probability of experiencing a delay or failure to restart the heart pump after it stops, Medtronic said in the notice.
- Since pulling the device from the market in June 2021, Medtronic has recalled it multiple times for problems including welding defects and batteries that failed to hold a charge.
Dive Insight:
Medtronic’s HVAD system is used in end-stage heart failure patients who are waiting for a heart transplant. The device consists of a pump implanted near the heart as well as a controller to manage the pump’s speed and function.
Medtronic previously identified two other subgroups of patients that face an increased likelihood of pump problems. One group, with 38 people, faced a 2.7% increased risk, while the other, with 17 people, faced a 31% increased risk.
Medtronic added a third subgroup after determining the pump failure rate for that group of patients had increased over time. All three groups of pumps flagged by the company share the same unnamed supplier, while other pumps that weren’t identified as having an increased risk were made by a different company.
In an FDA notice on Friday, the agency said that patients in one of the identified subgroups should contact their VAD coordinator if they need to make a controller exchange, so that it can take place in a clinical setting.












