About nine months after Medtronic won FDA approval for a drug-coated balloon to help maintain dialysis access in end-stage renal disease patients, the New England Journal of Medicine this week published the six-month results supporting that marketing authorization.
The renewed spotlight for the Medtronic-funded research comes as the vascular access procedures in which doctors were starting to adopt the IN.PACT AV device are seeing recovery after the pandemic's effects sparked a drop in procedure volume in March and April. At the same time, the virus has highlighted the importance of maintaining care for ESRD patients, who are in a higher-risk category, while minimizing time in and out of healthcare facilities.
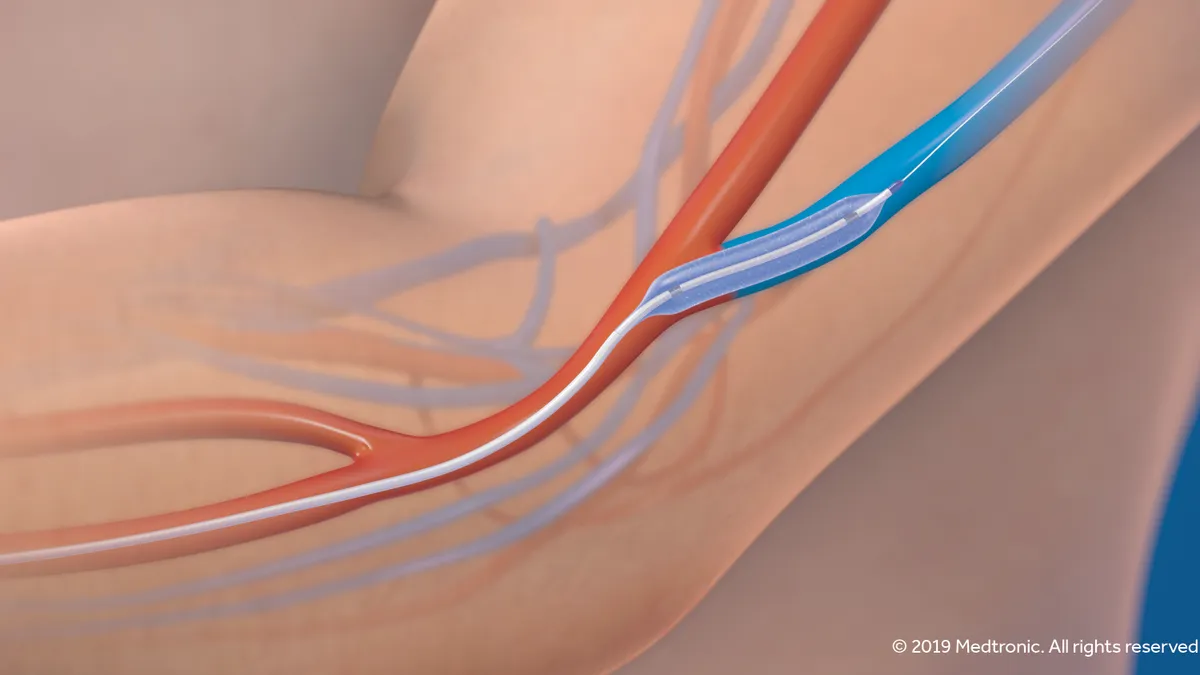
Prior to beginning hemodialysis treatment, ESRD patients must undergo a vascular access procedure to connect to a dialyzer. One option is to create an arteriovenous (AV) fistula, the connection of an artery to a vein that allows for wider blood flow, enabling better long-term access than some alternatives. The Medtronic balloon is meant to help maintain those AV fistulae better than standard percutaneous transluminal angioplasty.
Just this week, CMS updated guidance urging healthcare providers to carry out vascular access procedures "crucial" for ESRD patients on dialysis, including the establishment or repair of AV fistulas. CMS said that following guidance earlier in the pandemic to defer nonessential planned surgical procedures, it heard from providers about difficulties scheduling these vascular access procedures.
"We absolutely heard the confusion — in fact it was very clear that these procedures initially were not considered emergency procedures," said Mark Pacyna, VP of Medtronic’s peripheral vascular business. But after a downturn in U.S. procedures in March and April, "we think that we're seeing a lot of adoption in this technology because it really does benefit these patients to keep them on their dialysis treatment, to keep those fistalue working normally, and frankly, to keep them out of the healthcare system" amid COVID-19, Pacyna said.
The 330-patient IN.PACT AV Access Study, results of which first shared last fall at the Cardiovascular and Interventional Radiology Society of Europe annual meeting, met both its safety endpoint and its efficacy endpoint, which assessed target-lesion primary patency, specifically freedom from clinically driven target-lesion revascularization or access-circuit thrombosis in the six months following the procedure.
At the six-month mark, patients receiving the Medtronic device had a primary patency, or remaining open and unobstructed, rate of 86.1% compared to 68.9% in the PTA control group. Extended through 210 days, the rate among the Medtronic device group was 81.4%, to the control group's 59%.
At that same six-month readout, patients with the Medtronic balloon required 56% fewer re-interventions to maintain lesion patency compared to patients in the control group. By comparison, the LUTONIX AV trial that supported the 2017 approval of C.R. Bard's product, now sold by BD, found that recipients of the Lutonix balloon had 31.3% fewer re-interventions at six months versus patients receiving PTA.
Abbott has also made moves to bring a device to market, having previously secured the option to negotiate an agreement for an in-development AV fistula drug-coated balloon product from Minneapolis-based medtech Surmodics.
The already broad understanding of, and comfort with, balloon angioplasty as a technique across medical specialties is a boon to future adoption, said principal investigator Robert Lookstein, executive vice-chair in the Mount Sinai Health System's department of diagnostic, molecular, and interventional radiology.
As for additional study beyond the premarket trial setting, "I think everybody agrees that it does need to be extrapolated to more of a real-world experience to understand who is ideally suited for it," Lookstein said.
Medtronic is still in the process of working out the details of a registry with FDA, Pacyna said.
Controversy lingers
The IN.PACT AV balloon builds on a previously approved variation of the device, known as Admiral, intended to help treat femoropopliteal disease.
Just over a year has passed since FDA publicly scrutinized the use of paclitaxel, the drug used with IN.PACT AV, in balloon and stent treatment of that peripheral artery disease, following identification of a safety signal suggesting higher late mortality among patients receiving the drug via those devices. Since an FDA advisory committee meeting last summer, Medtronic and other market players BD, Boston Scientific, Cook Medical and Philips have updated their labels to reflect the safety warning.
Pacyna said work is still going on behind the scenes to make sure the company can update FDA over the coming months. "Physicians continue to have questions ... I would say that the controversy is not over, but the stakeholders have made significant progress over the past year and continue to work closely together to solve that."
In addition, Pacyna said Medtronic continues to look at other indications for the IN.PACT balloon platform, and noted the company's ongoing interest in a below-the-knee (BTK) application. BD has also sought a BTK indication, but FDA sent the company back to the drawing board last year. The agency reportedly told BD the clinical evidence in its PMA submission was not sufficient.