Dive Brief:
- Medtronic shared new data showing its extravascular implantable cardioverter defibrillator (EV ICD) met safety and efficacy endpoints, with a defibrillation success rate of 98.7%.
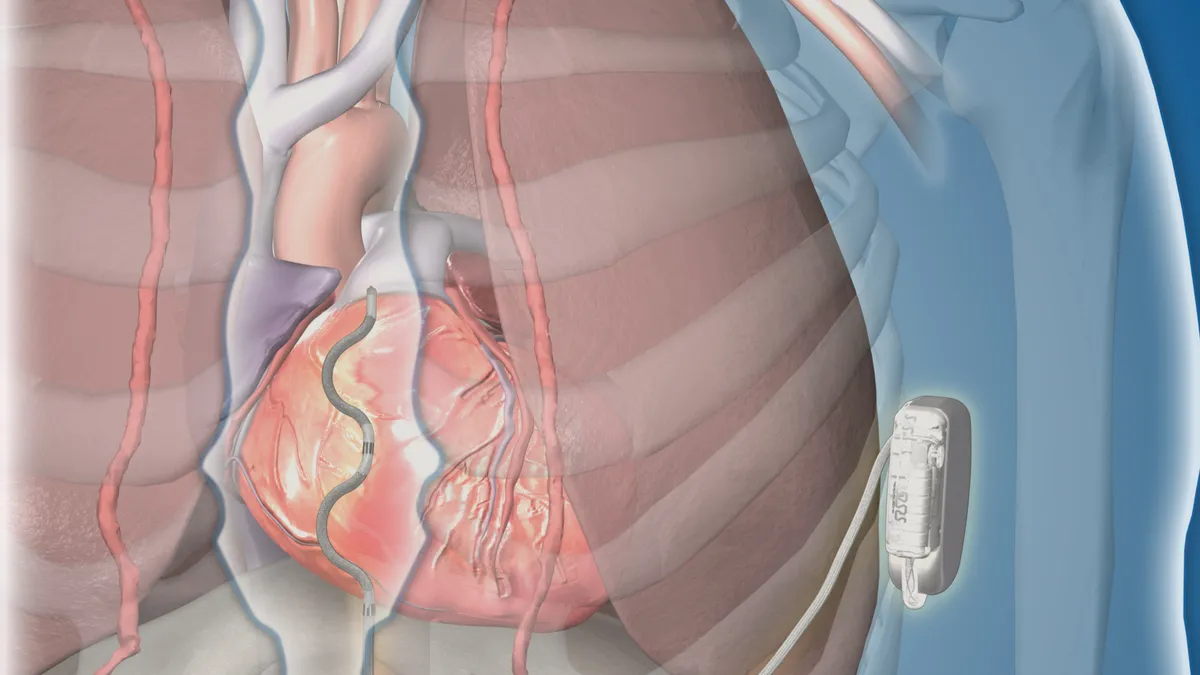
- The device is designed to treat fast heart rhythms that can lead to sudden cardiac arrest. The lead is placed under the sternum, which Medtronic says helps avoid complications that can come with leads in the heart and the veins, such as infection or vessel occlusion.
- The data could position Medtronic’s device as a competitor to Boston Scientific’s subcutaneous implantable defibrillator (S-ICD) system, which got approval from the Food and Drug Administration in 2012, J.P. Morgan analysts wrote in a Sunday research note. Medtronic is waiting on a CE Mark and expects to get FDA approval next year, CEO Geoff Martha said in the company’s most recent earnings call.
Dive Insight:
Medtronic presented the study results at the European Society of Cardiology Congress in Barcelona on Friday. Of 306 patients, 98.7% had successful defibrillation at the time the device was implanted. After six months, 92.6% were free from major system or procedure-related complications, according to data published in the New England Journal of Medicine.
A total of 25 major complications were observed in 23 patients, and a total of 29 patients had received 118 inappropriate shocks for 81 arrhythmic episodes.
During the 10.6-month mean follow-up period, eight systems were removed without a replacement device.
J.P. Morgan analysts wrote that, “overall the results are a positive for Medtronic, look to be competitive relative to Boston’s S-ICD, and should help to modestly expand the market …”
Still, they wrote that they expect Boston Scientific to remain a leader in the market for non-transvenous defibrillators, given that it has a big head start and a long history in the market.
Comparing data for the two devices, Medtronic’s EV ICD overall looks to be competitive relative to Boston’s S-ICD, the analysts wrote. despite caveats with cross-trial comparisons, especially since Medtronic’s was a single-arm trial.
The analysts noted the higher rate of inappropriate shock at 10.6 months in the Medtronic study, at 9.7%, compared to 2.4% at one year for Boston Scientific’s Emblem MRI S-ICD in its UNTOUCHED study. Still, they said that most of the incidences occurred earlier in the study, and many shocks were avoided with the use of anti-tachycardia pacing.