Dive Brief:
- Medtronic has linked its transcatheter aortic valve replacement (TAVR) device to a lower rate of valve dysfunction than Edwards Lifesciences’ rival product in a subpopulation of patients. The results were released Sunday at the American College of Cardiology’s (ACC) Annual Scientific Session.
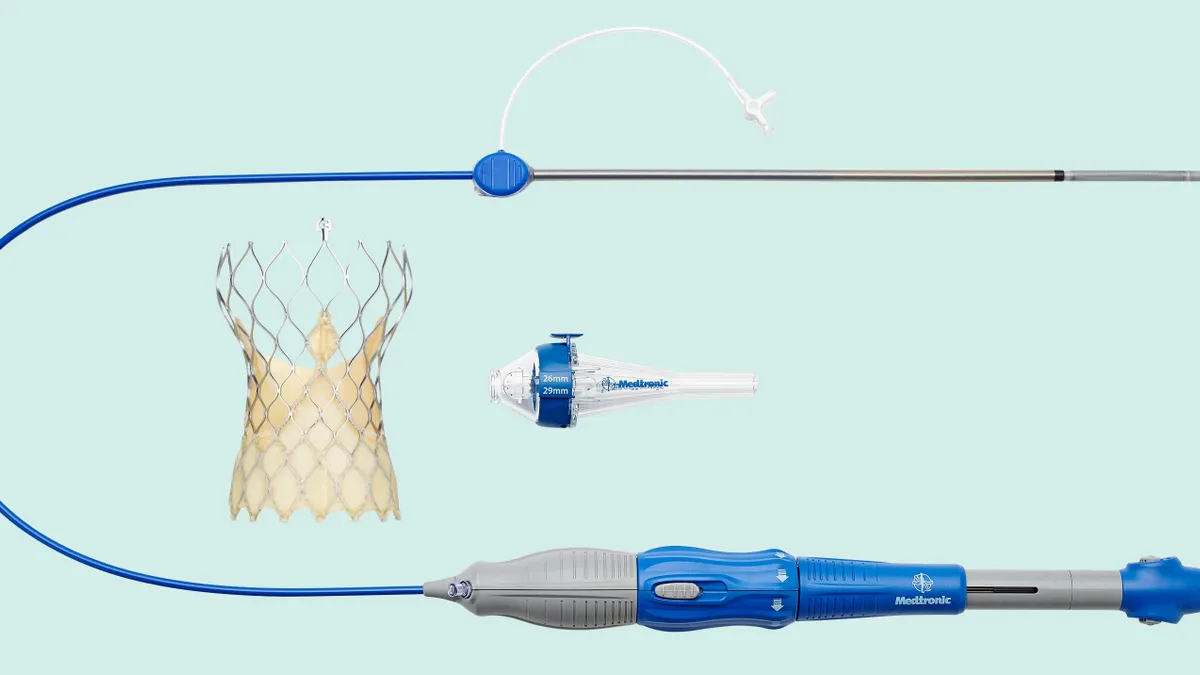
- The results, also published Sunday in The New England Journal of Medicine, show 9.4% of patients with small aortic annulus had bioprosthetic-valve dysfunction through 12 months after treatment with Medtronic’s Evolut valve, compared with 41.6% of patients who received Edwards’ Sapien device.
- Medtronic estimates 35% of Edwards’ valves are used for small annulus patients, most of whom are women, and is aiming to capture that piece of the market. The company also sees a chance to expand the market by targeting women who are currently underdiagnosed and undertreated.
Dive Insight:
The study specifically targeted patients with small aortic annuli, which Medtronic said are primarily women. The aortic annulus is the transition point between the left ventricle of the heart and the aortic root.
Cardiologists identified potential advantages to supra-annular TAVR valves, such as Medtronic’s Evolut, in patients with small annuli in a paper published in 2020. Later that year, Medtronic began a head-to-head clinical trial to compare its Evolut valves to Edwards’ balloon-expandable Sapien devices in the patient population.
Investigators randomized more than 700 patients, nearly 87% of whom were women, to receive Evolut or Sapien valves. After one year, the rate of valve dysfunction was significantly lower in the Evolut group, causing the trial to hit one of its co-primary endpoints.
The second co-primary endpoint, which Medtronic tested for noninferiority, was a composite of death, disabling stroke or rehospitalization for heart failure. Evolut had a small numerical advantage over Sapien on that endpoint.
Nina Goodheart, Medtronic’s president of structural heart and aortic, said on an investor call on Sunday that “everyone expected a positive trial” but claimed physicians were “shocked” about the magnitude of superiority. Goodheart asked why women should have to tolerate valve dysfunction “when you have a treatment option that doesn't have it?” and expects that thinking to shape discussions about valve selection.
“Every physician has to start their heart team meetings next week asking ... ‘In this patient, based on this randomized clinical trial, a landmark trial, why would you not put Evolut in?’ Physicians are going to have to rationalize that. It's going to be hard to rationalize after we see this data,” Goodheart said. “So, do I think we're going to see some meaningful shifting? Yes. Do I think it's going to take some time? Of course.”
CEO Geoff Martha added that Medtronic has “some really simple messaging” on the head-to-head performance of the devices, and the company will “make sure that clinicians and patients get this message and clearly understand the benefits.”
J.P. Morgan analysts were critical of Medtronic's results, writing in a note to investors that the trial outcomes “deviate significantly from what’s seen in clinical practice and prior trial results for Sapien.” The analyst noted two key factors on the results: the trial was designed at the discretion of Medtronic, without input from the Food and Drug Administration, and there “appears” to be an under-sizing of Edwards’ Sapien valves, which could explain the real-world outcomes.
“While the headline reads positive for [Medtronic,] we don’t expect today’s results to shift clinical practice or TAVR share, and could actually lead to a relief rally in [Edwards’] shares, in our view,” the analysts wrote.
The head-to-head trial was one of two main areas of focus for Medtronic at ACC. The other was Evolut FX+, a new TAVR device that the FDA approved in March. Medtronic plans to start cases in the spring ahead of a full market release in the summer, Goodheart said.
Evolut FX+ is designed to have the radial strength and hemodynamics of its predecessors and address a perceived issue with coronary access, she explained.
“As [patients] continue to get younger, physicians have said they want to be able to make sure that they can get to the coronary arteries. There was a perception that it was a little bit harder to do it through this tall frame,” Goodheart said. “We have fixed that. I think we have put that now to rest with FX+.”