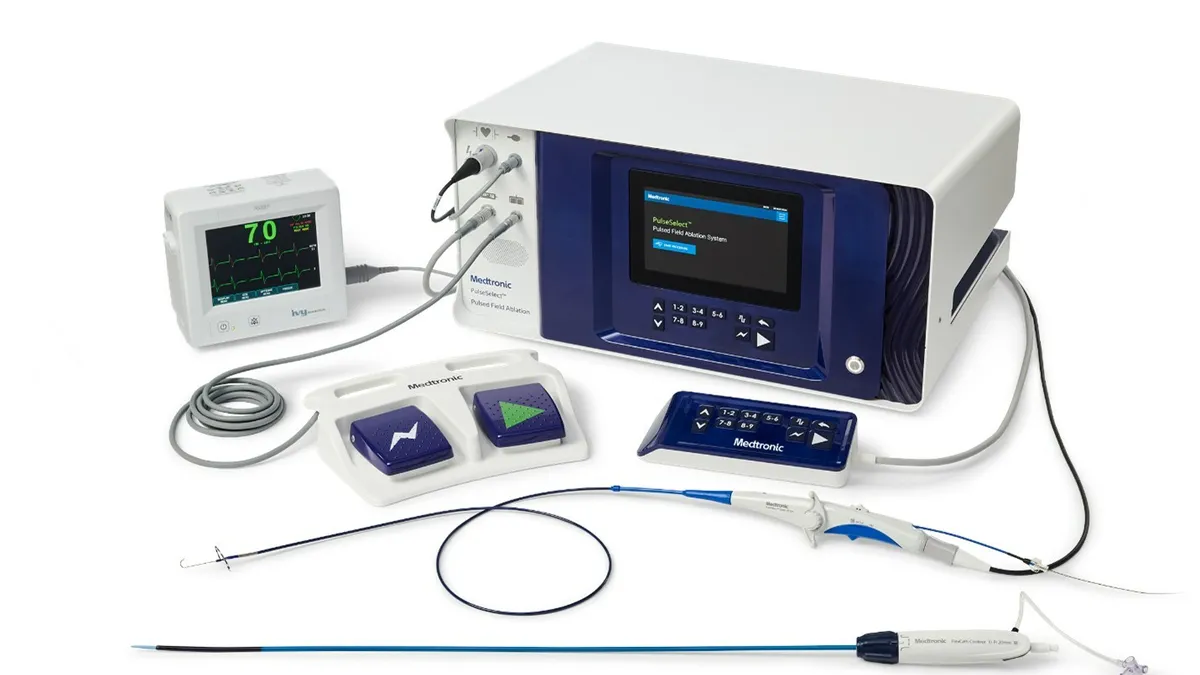
Medtronic executives touted the early demand for its pulsed field ablation (PFA) system on a Thursday earnings call, following Boston Scientific’s lead of hyping the new technology for investors as products hit the market.
Sean Salmon, president of Medtronic’s cardiovascular portfolio, told analysts that demand in the U.S. is “moving pretty fast” and “we're catching that wave, obviously, with our first entry.”
PFA is a new treatment for atrial fibrillation, a type of irregular heartbeat that can lead to heart failure or strokes. The catheter-based systems use non-thermal electrical pulses to target cardiac tissue, compared with the heat-based and extreme cold methods used in radiofrequency ablation and cryoablation, respectively.
The PFA market has received a lot of attention from top medtech companies and Wall Street analysts this year, after Medtronic and Boston Scientific recently gained the first Food and Drug Administration approvals for the technology.
Early glimpses of product market performance and physician feedback have also spurred the excitement building around the technology. In April, Boston Scientific CEO Mike Mahoney called the company’s Farapulse system the “most transformational product that I’ve seen in my career with the company.” Meanwhile, Truist Securities analyst Richard Newitter wrote that feedback from electrophysiologists at the recent Heart Rhythm Society annual meeting suggests a very steep adoption curve “right out of the gate.”
Medtronic has now added to the momentum with a first look at the system’s performance in the U.S. CEO Geoff Martha said on the call that PFA would be a big product category for the company in its fiscal year 2025, which began in April.
The company did not provide specific sales figures for its PulseSelect PFA system, similar to Boston Scientific. However, Medtronic highlighted on the call and in earnings materials that declines in its cryoablation business were “more than offset by strong growth” in PFA.
Martha added that the cardiac ablation solutions business grew 21% sequentially in Medtronic’s fiscal fourth quarter, fueled by the recent addition of PFA. The cardiac rhythm and heart failure unit, which includes the ablation segment, grew year over year by 1.3% to $1.59 billion in the quarter.
After receiving FDA approval for its PulseSelect PFA product in December, Medtronic is looking to get a second system to market, called Affera, which combines an ablation system with cardiac mapping.
Medtronic still has work to do for its PFA business overall. Salmon told investors the company is working to get international approvals, expand capacity and scale up manufacturing to meet demand, which is “something we’ve put a lot of effort toward.”
New Hugo clinical trials
Medtronic also announced the launch of clinical studies in hernia and gynecology procedures for its Hugo soft tissue surgical robot, with plans to use the data to support FDA submissions. The company is already pursuing a urology indication for the system.
“[It’s] critical that we are now operating across multiple indications, so that as we come into the market, we can have a series of launches across each of those to capture larger and larger pieces of the market,” said Mike Marinaro, Medtronic’s president of the medical surgical portfolio and surgical operating unit.
Medtronic, along with J&J, are trying to chase down market leader Intuitive Surgical with soft tissue robotic systems of their own. However, there are still questions as to how well they can compete as their systems continue to lag behind, and Intuitive just launched a new generation of its da Vinci robot.
Marinaro would not estimate when Medtronic would submit for Hugo’s urology indication but added that “we are nearing completion” of the trial.