Two years after hitting Medtronic with a warning letter for product safety problems with its diabetes group, the Food and Drug Administration lifted the warning on Tuesday.
The regulatory change allows Medtronic to bring products to market in the U.S. more quickly, ending a period of delay for the company.
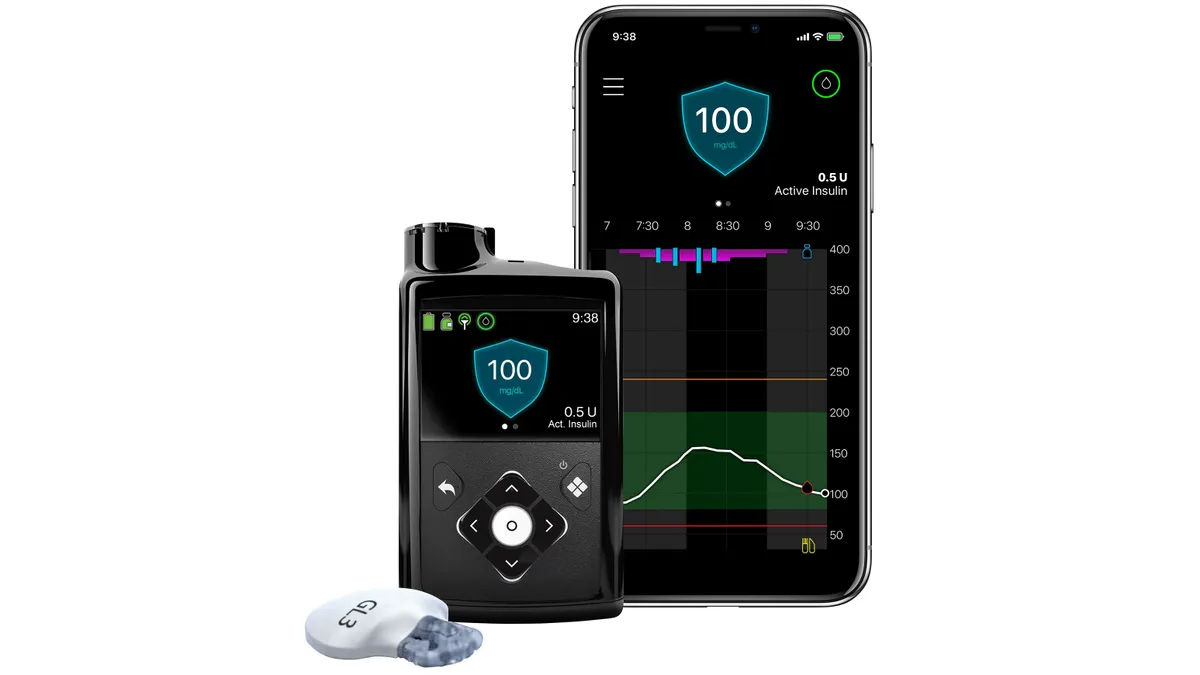
Medtronic received the warning letter from the FDA in 2021 after finding problems with the retainer rings for its MiniMed 600 series insulin pumps could result in users receiving an incorrect dose of insulin. The warning letter said the company did not appropriately classify the patient risk, and did not initiate a recall, despite thousands of medical device reports made over three years related to the problem.
In a statement on Tuesday, Medtronic said the FDA lifted the warning letter after ongoing remediation from the company, and that all regulatory restrictions associated with the warning letter have been resolved.
"We're very thankful to the agency for working with us so collaboratively to ensure we're able to work as quickly as possible to address the needs of the diabetes community," Que Dallara, who was named president of Medtronic Diabetes last year, said in a statement.
After Medtronic’s MiniMed 780G insulin pump received FDA approval on Friday, analysts speculated that the warning letter would be lifted soon. That would “make possible more timely regulatory decisions on MDT's new diabetes devices that are awaiting US approval, including its Simplera sensor,” TD Cowen analysts Joshua Jennings and Eric Anderson wrote in a research note on Friday.
The FDA did not immediately respond to requests for comment at the time of publication.