In the multibillion-dollar market for diabetes technology, Medtronic’s U.S. diabetes sales have declined in the last four years as the company grappled with regulators and competitors rolled out new devices.
With two strokes of a pen in late April, the Food and Drug Administration has given Medtronic a chance to change that. A warning letter over quality issues at its Northridge, Calif., diabetes device facility was lifted after two years, and the agency has approved Medtronic’s newest insulin pump, the MiniMed 780G, with its Guardian 4 sensor.
Still, is that enough to help the company regain lost market share?
“Despite Diabetes representing one of the most attractive MedTech markets, Medtronic’s sensor technology and tubed pump form factor still lags behind the competition,” J.P. Morgan analyst Robbie Marcus wrote in an April research note, after Medtronic received approval for the new insulin pump. “With Insulet and Tandem soon to be integrating with both Dexcom and Abbott’s newest generation sensors, it will be a steep hill to climb for Medtronic to recapture share.”
Medtronic’s diabetes sales hit a four-year low of $2.34 billion in its 2022 fiscal year. So far, the segment is on pace to decline about 13% in the company’s 2023 fiscal year, which ends in April, BTIG analyst Ryan Zimmerman wrote in a research note.
“It will be a steep hill to climb for Medtronic to recapture share.”

Robbie Marcus
Analyst, J.P. Morgan
Medtronic CEO Geoff Martha played up the prospects for the diabetes business in the company’s most recent earnings call in February, even as the company is in the process of spinning off some of its slower-growing business segments.
Medtronic will “continue to invest heavily” in its diabetes care business, with “multiple programs under development,” and with “the intent of restoring strong growth of our important diabetes franchise over the coming years,” Martha said.
Still, the company’s competitors, who were able to bring new technologies to market while Medtronic resolved regulatory problems, have a significant head start. That leaves Medtronic facing an arduous task, according to analysts.
Declining U.S. market share
The U.S. market makes up about 38% of Medtronic’s total diabetes sales and insulin pumps and continuous glucose monitors account for about 63% of the total, Zimmerman wrote.
Medtronic’s U.S. diabetes business has been on the decline since 2019, even as international sales grew.
Medtronic’s competitors, in the meantime, have grown their businesses. Among companies that make continuous glucose monitors, Abbott’s diabetes business brought in $4.76 billion in sales last year and Dexcom reported revenue of $2.91 billion. Insulin pump-makers Insulet and Tandem Diabetes Care reported revenues of $1.3 billion and $801.2 million, respectively.
In addition, competitors have been refreshing their offerings as Medtronic stalled.
Last year, Insulet brought a new tubeless insulin pump to market, and Dexcom and Abbott launched new continuous glucose monitors. The newer devices had some features that Medtronic’s older devices lacked, including continuous glucose monitors that didn’t require twice daily calibration using a fingerstick, and insulin pumps with an auto bolus correction feature to avoid late night alarms. These are features that Sanjoy Dutta, chief scientific officer of diabetes research nonprofit JDRF, said “are almost very common these days.”
“And so, there was a period where people were probably switching” to other brands, Dutta said.
All of this happened during the height of the COVID-19 pandemic, when patients were worried about their health and whether access to insulin supplies would be disrupted, he added.
“A warning letter … is never going to be without any speculation, discussion or even concern,” Dutta said, adding that “there are quite a few options in the device world right now.”
Warning letter resolution
Resolving the warning letter removes regulatory restrictions on future product approvals, which allows Medtronic to bring new diabetes devices to market faster, RBC Capital Markets analyst Shagun Singh wrote in a research note. It should also save the company additional money it had been spending on its quality assurance system, though Medtronic has not specified the amount, Singh wrote.
Medtronic received the warning letter in December of 2021, flagging quality control problems after an inspection of the company’s diabetes headquarters in California. The inspection was related to recalls of its MiniMed 600 series insulin pumps, where broken or missing retainer rings could cause users to receive the wrong dose of insulin.
The warning letter said Medtronic did not appropriately classify the risks for the devices, and waited three years until 2019 to initiate a recall, despite thousands of medical device reports being made during that time period.
The company had to pass a re-inspection before the warning letter was lifted.
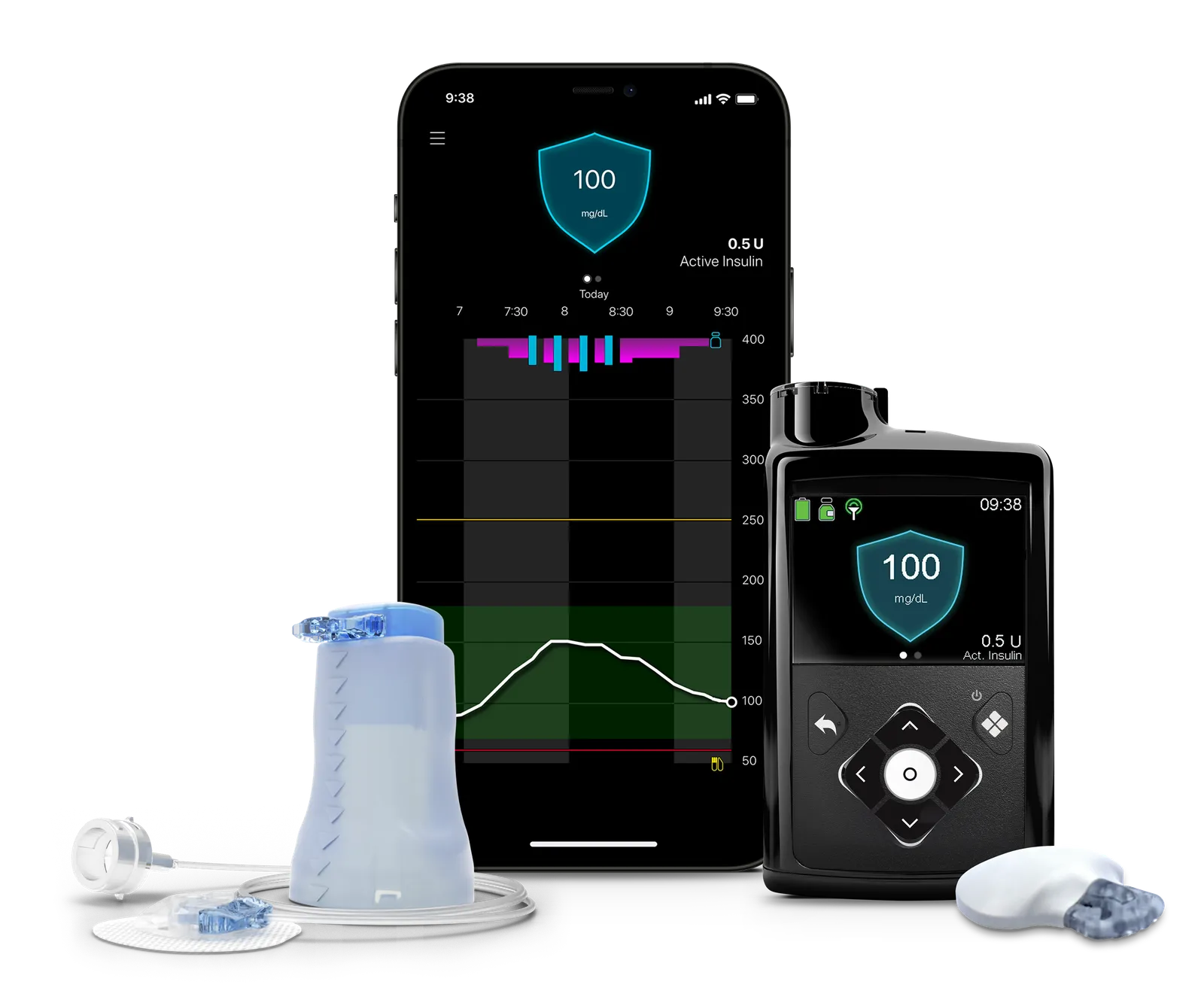
New device approval
One of the new products Medtronic hopes will drive future growth is its MiniMed 780G pump, which received FDA approval in late April paired with its Guardian 4 continuous glucose monitor. Although the device was approved in Europe in 2020, and submitted to the agency for approval in early 2021, it got held up for two years in the wake of the warning letter.
"We’re confident that we’ll be able to replicate the success we’ve experienced in other regions where we’ve launched the MiniMed 780G system."

Que Dallara
President of Medtronic’s diabetes unit
Medtronic is counting on the uptake of the device in the U.S. to be similar to Europe, where it is available in 90 countries and has driven growth in continuous glucose monitors being paired with the insulin pump, according to the company’s recent earnings call.
“There’s been a lot of pent up interest in our system and the response to the FDA approval has been overwhelmingly positive. We’re confident that we’ll be able to replicate the success we’ve experienced in other regions where we’ve launched the MiniMed 780G system,” Que Dallara, who was named president of Medtronic’s diabetes unit last year, wrote in an emailed statement.
Currently, Medtronic’s pump is approved to work with its Guardian 4 sensor. Typically, its devices have not been interoperable with other companies’ pumps and sensors, because it’s the only company that has all three components of a hybrid closed-loop system: a CGM, insulin pump and algorithm, Dutta said.
The company is also working on a new, disposable CGM called Simplera, that’s about half the size of the Guardian 4. It submitted the new device for a CE Mark last summer and to the FDA earlier this year, RBC’s Singh wrote.
The company “would likely need a smaller sensor like Simplera in order to compete more effectively, and even then it could be an uphill battle as the company has seemingly lost credibility among patients and physicians,” Singh added.
How it compares to competitors
Medtronic plans to start taking pre-orders for MiniMed 780G on May 15, for both new and existing customers, Dallara said. It will start shipping the devices, and upgrading existing customers in June. People who are currently using Medtronic’s previous insulin pump model, the 770G, will be able to access the new technology through a free software upgrade, according to the company’s website.
Medtronic is relying on some unique features and recent study results to help set the system apart from competitors. For one, the 780G features the lowest glucose target setting, 100 mg/dL, of any device currently on the market, which more closely mirrors the average glucose of someone not living with diabetes, Dallara wrote. Users can pick from three different glucose targets. It also can deliver correction doses every 5 minutes, more closely mimicking the function of a healthy pancreas, and adjusting for missed meal boluses or underestimated carbs, Dallara added.
“The compelling testimonials from customers around the world, the high product satisfaction users are experiencing, the data from both our ADAPT study and real-world experiences that demonstrate significantly improved Time in Range and ease of use – these should give us a read on how we expect it to perform in the U.S. where it’s now approved,” Dallara said, when asked how the company plans to regain market share for its diabetes business.
Medtronic’s Guardian 4 sensor no longer requires twice daily calibration using fingersticks, like its older CGM did. An extended infusion set for the pump allows it to be worn for twice as long — seven days — before changing infusion sites.
“I think that is a very big deal,” JDRF’s Dutta said, adding that for the past few decades most pumps have required the site to be changed every three days, which is not only a hassle but can also result in a buildup of scar tissue from inserting and removing the device.
At the recent Advanced Technologies & Treatments for Diabetes Conference in Berlin, Medtronic shared data from more than 60,000 people who used the device in Europe, “and the data looked very nice,” said Dutta.
Still, some physicians said they weren’t interested in switching patients to the new Medtronic pump because their patients are happy with the pumps they are using now, and other, smaller sensors are available than the G4, RBC’s Singh wrote, citing conversations with key opinion leaders.
“One of our [key opinion leaders] did mention that she may be interested in trying the offering because of more frequent boluses of the MiniMed 780G ([Medtronic] every hour vs. every 5 hours for [Tandem]) and low target range of 780G, which is a benefit for some, e.g., pregnant women,” Singh wrote in a research note. “We heard from several [key opinion leaders] that there was a very sticky/loyal base of [Medtronic] customers on MiniMed, but their technology disadvantage vs. newer competitors made many of them switch.”
Medtronic has not yet said whether the lifting of the warning letter or the new device will change its financial forecasts for its 2024 fiscal year, which starts in June.




















