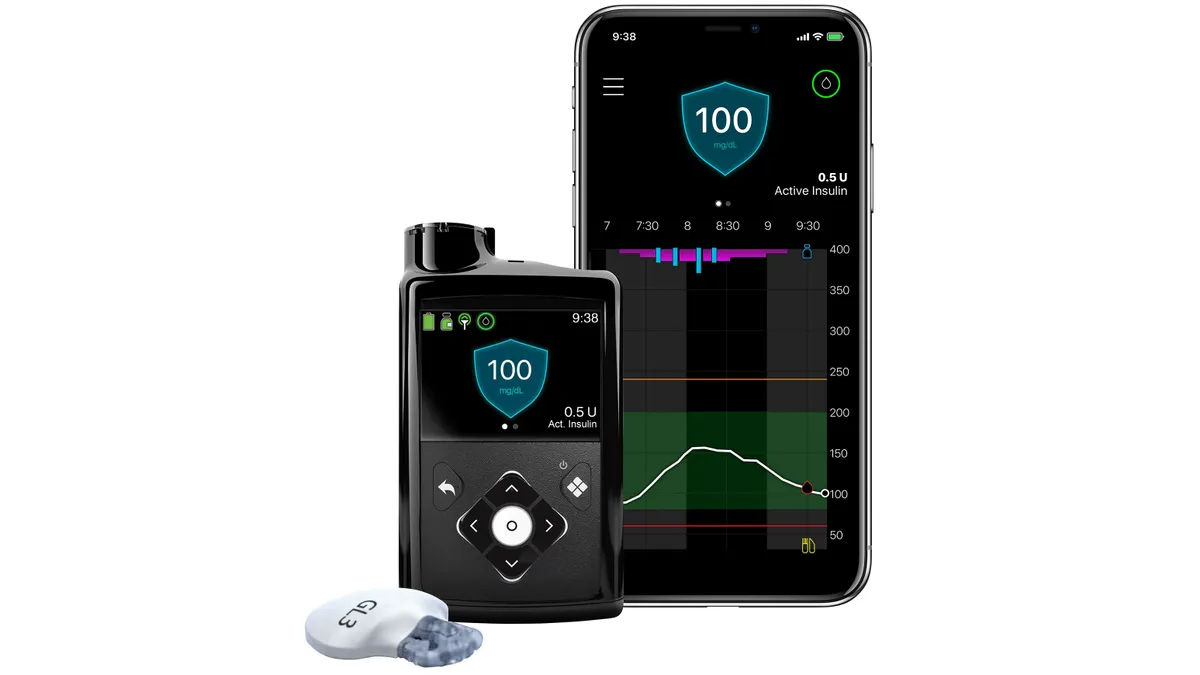
Dive Brief:
- Medtronic’s MiniMed 780G hybrid closed loop system has provided sustained improvements in the control of Type 1 diabetes for one year, according to a study.
- The update showed that the improvements seen after six months persisted in the follow-up period, and revealed that participants who initially received multiple daily injections (MDI) of insulin had better blood glucose control after switching to MiniMed.
- Medtronic framed the data as evidence that patients should receive automated insulin delivery earlier, rather than starting on MDI and switching if they have problems. However, the company has yet to bring the benefits to U.S. patients because of a regulatory delay in approving the 780G.
Dive Insight:
In September, Medtronic released six-month data that linked the MiniMed 780G to significant improvements in blood glucose levels and time in target range compared to MDI informed by an intermittently scanned continuous glucose monitor (CGM). The study provided evidence that using the 780G pump to automate insulin delivery provides better outcomes than manual injections.
Now, Medtronic has released data on what happened in the second part of the study. Patients randomized to use MiniMed 780G from the start of the trial continued to use the pump and retained the improvements they experienced over the first six months. Over the second six months, blood glucose, as measured by mean HbA1c, rose slightly, from 7.3% to 7.4%, and time in range slipped from 70.4% to 69.7%.
The bigger changes were seen in the cohort of patients who were in the MDI control group for the first part of the trial. In the six months after switching to MiniMed 780G, mean HbA1c fell from 8.9% to 7.5%, and mean time in range increased from 43.6% to 70.7%.
In a statement, Ohad Cohen, senior global medical affairs director for Medtronic Diabetes, said, “These results further strengthen the case for us to move beyond the CGM-first paradigm,” adding that “the MiniMed 780G system is taking care of users in ways that individuals just aren't able to with manual injections, even with the best of intentions.”
The message is resonating commercially, with the 780G driving 18% growth outside the U.S. at Medtronic’s diabetes unit in the most recent quarter. However, Medtronic is still in “active review” with the Food and Drug Administration about its filing to bring the device to the U.S. market. The authorization was delayed by regulatory problems at Medtronic's diabetes unit.