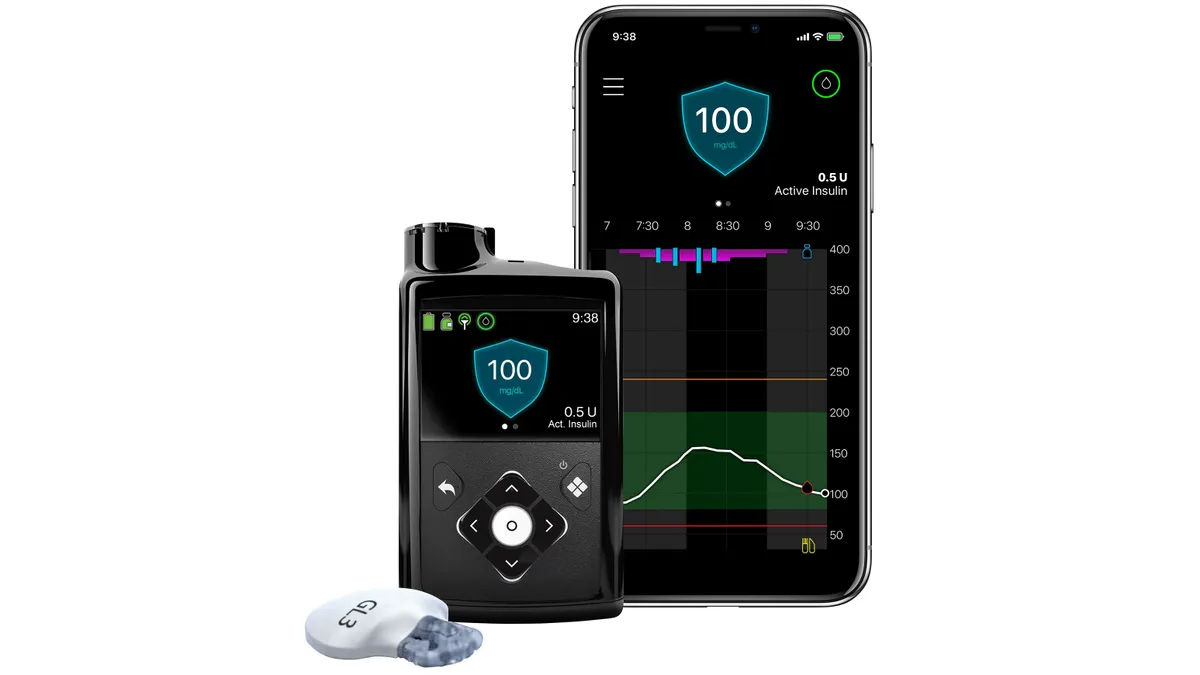
Dive Brief:
- Medtronic has won Food and Drug Administration approval for its MiniMed 780G, ending the long wait for authorization to sell the device in the U.S.
- Approval of the insulin pump was delayed by a warning letter the FDA sent to Medtronic’s diabetes department late in 2021. Since then, Medtronic has worked to overcome the regulatory barriers to launching a device that could help it compete against Insulet and Tandem Diabetes Care.
- In a note to investors, analysts at RBC Capital Markets called the FDA approval a “positive” for Medtronic that “should somewhat aid in a turnaround of its U.S. diabetes franchise.” However, the analysts think Medtronic has lost credibility and will need a smaller sensor to compete.
Dive Insight:
Medtronic’s fortunes have diverged inside and outside the U.S. since the warning letter. Internationally, where MiniMed 780G has been available since 2020, the company reported 18% growth in its diabetes division during its fiscal third quarter. However, diabetes sales fell in the U.S., a trend that Medtronic attributed to a lack of new products.
The question now is whether MiniMed 780G can reverse the trend. The insulin pump has a feature, SmartGuard, that automatically adjusts insulin dosing without the user inputting finger prick data and accounts for users forgetting to bolus dose or underestimating the carbohydrate content of a meal.
In a statement, Que Dallara, president of Medtronic Diabetes, said “mealtimes prove to be one of the biggest challenges for people living with type 1 diabetes” and explained how the latest version of MiniMed addresses the unmet need.
“A lot can happen to blood sugars in the span of an hour or even just a few minutes, so we've designed our system for real life – the algorithm adapts to the user and helps compensate for everyday challenges that are quite common around mealtimes. We built in features informed by extensive customer feedback and we're excited to deliver a system with ease of use at the forefront,” Dallara said.
Earlier this year, Medtronic presented clinical trial data that showed improvements in glucose control persisted for at least one year in users of the MiniMed 780G hybrid closed loop system. Participants who received multiple daily injections over the first half of the trial switched to the MiniMed system for the next six months of the study. The patients’ blood glucose control improved after they switched.
Medtronic has already filed for approval of its new Simplera sensor, which is around half the size of the Guardian 4 sensor used in MiniMed 780G today. Based on talks with doctors, RBC analysts told investors Medtronic “would likely need a smaller sensor like Simplera in order to compete more effectively, and even then it could be an uphill battle as the company has seemingly lost credibility among patients and physicians.”