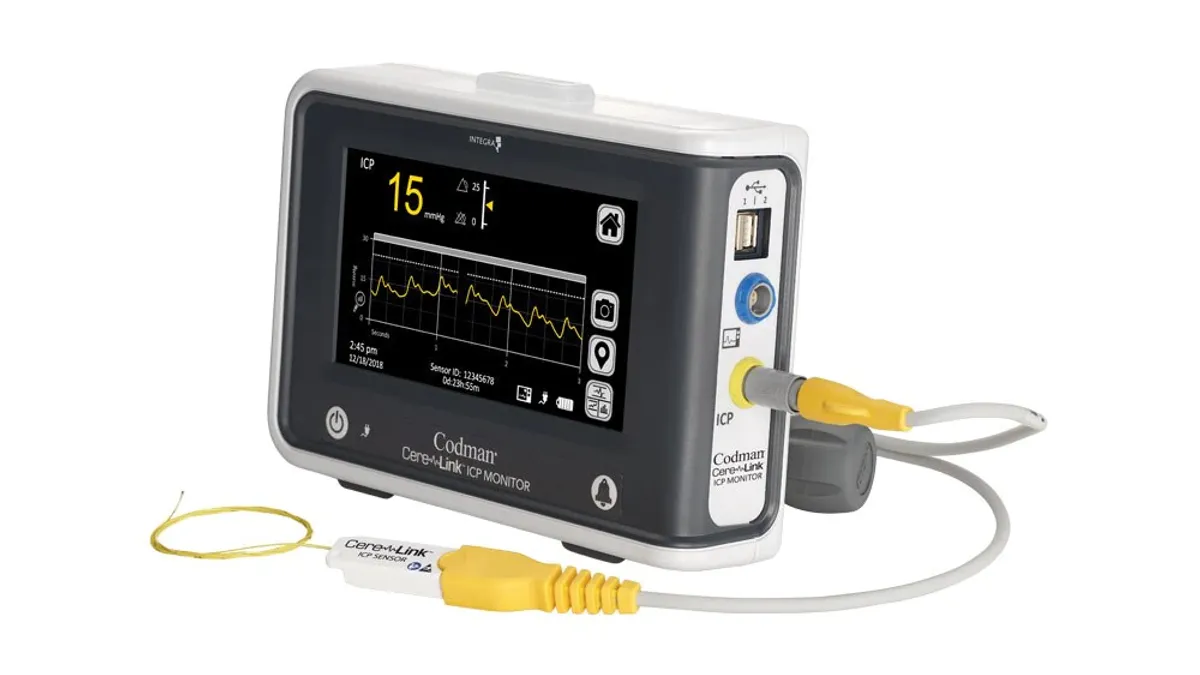
Dive Brief:
- A problem that prompted Integra LifeSciences to immediately remove all CereLink intracranial pressure (ICP) monitors has been described in a report of the death of a patient.
- In its notice about the Class I recall, the Food and Drug Administration said one of the at least 68 Medical Device Reports associated with the problem describes the death of a critically injured patient.
- The report said the team caring for the patient had to replace the ICP sensor in a malfunctioning CereLink ICP Monitor. However, Integra determined that the death was unrelated to the device malfunction.
Dive Insight:
Integra disclosed the immediate global voluntary removal of its CereLink ICP monitors last week. At the time, the company said it initiated the action after receiving customer reports about monitors that gave out-of-range pressure readings. Integra spoke to agencies including the FDA and, while the problem was seen at a low rate at a limited number of sites, decided to remove all CereLink monitors from the field.
The FDA now has provided more information about the recall of 388 devices in the U.S. Integra had received 105 global complaints associated with the recall as of the end of July, according to the agency. The agency’s review of its own records revealed at least 68 Medical Device Reports as of Aug. 24.
The 68 reports include descriptions of patient injury and the one death. The reports in the FDA’s public database include a description of a problem with the CereLink pressure reading that led to a “significant surgical delay” of two to four hours.
Integra expects a $9 million returns provision related to the recall in the third quarter. Factoring the provision into the financial outlook led the company to lower its third-quarter sales forecast to $374 million to $382 million.