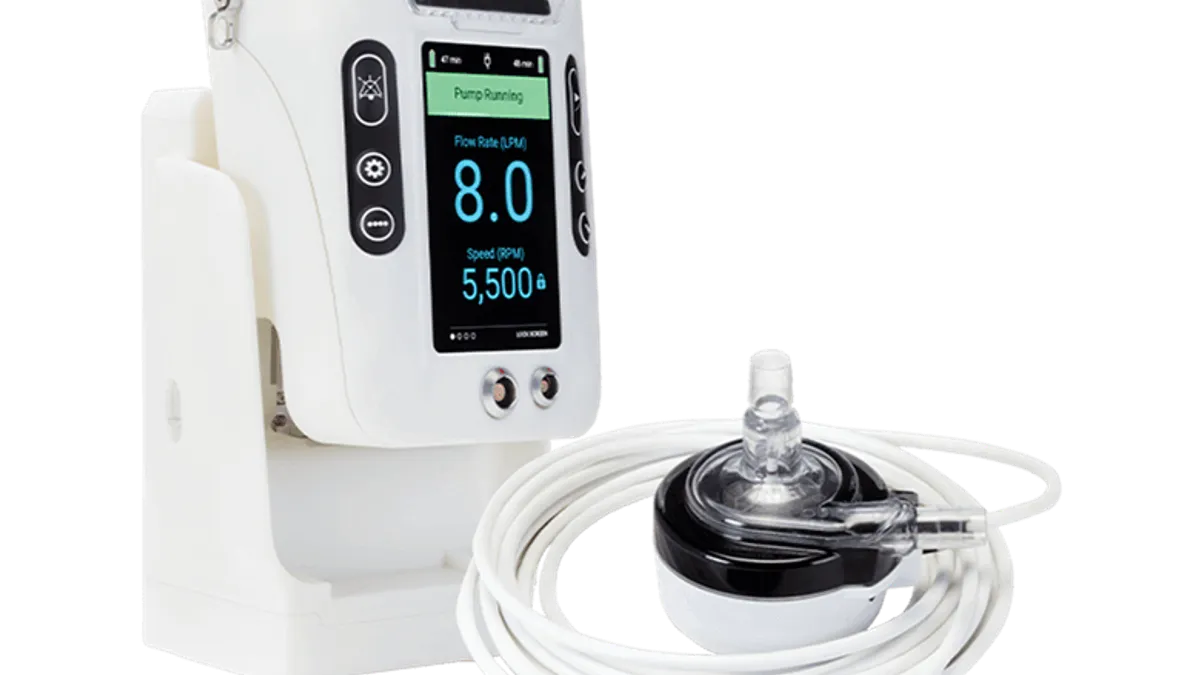
LivaNova is recalling some of its LifeSPARC circulatory support controllers for a software update to fix a malfunction that could cause the devices to stop working. The FDA announced earlier this month that it had determined the recall was a Class I event, the most severe kind, and capable of causing the death of patients.
The recall includes 589 LifeSPARC controllers that were distributed from December 2019 to November 2022.
The devices pump blood through an external circuit to support patients during surgical procedures. They’re made by London-based LivaNova, a company that specializes in life support.
Last July, LivaNova first recalled the device because of the software malfunction that could put the device into critical failure mode, stopping the pump. It had received 66 complaints about the problem, and reports of two injuries, according to an October notice by the Food and Drug Administration.
The latest recall was issued because a software update that addresses this malfunction is available for the device controller. Customers will be contacted by a third-party medical device repair firm to install it.
In the meantime, LivaNova recommends hospitals ensure a backup controller is available, in the event that the device needs to be replaced during a critical failure.