Dive Brief:
- Levita Magnetics has received 510(k) clearance for a minimally invasive surgical platform for use in abdominal procedures.
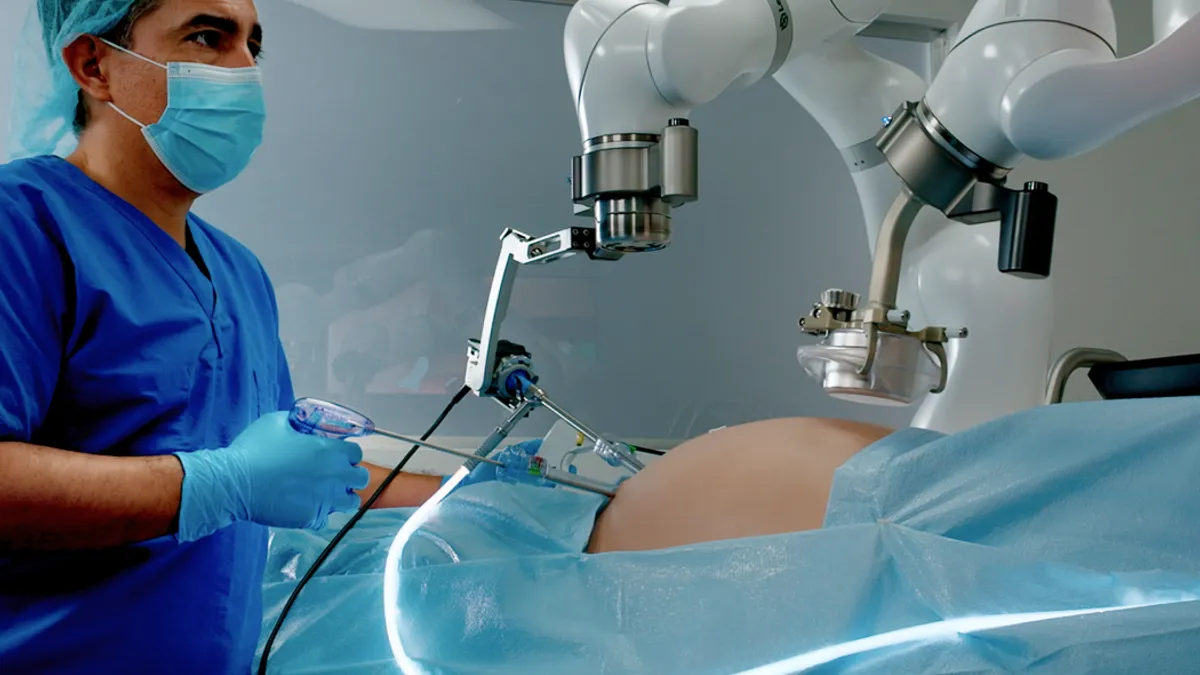
- The U.S. Food and Drug Administration cleared the surgeon-controlled arm developed by Levita earlier this month. Levita said the platform, branded MARS, should reduce the need for an additional assistant while using its magnetic surgery technology.
- Levita, which raised $26 million to bring MARS to market last year, received de novo clearance for its magnetic surgical system in 2016 and has linked the approach to improved outcomes.
Dive Insight:
Levita identified magnets as a way to overcome challenges device developers and surgeons encountered as they tried to make laparoscopic procedures less invasive and minimize damage to the patient. Efforts to reduce the number and size of incisions hindered procedures, but magnets offered an alternative with the potential to cause less pain, fewer scars and a faster recovery.
In magnetic procedures, a device is put inside the patient and controlled through the abdominal wall by an external magnet. Surgeons have used magnetic devices in procedures to retract the liver and remove the gall bladder.
Levita sees the addition of a robotic arm controlled by the surgeon as the next evolution of the platform. Adding a robotic arm could give surgeons control of both the laparoscopic view and magnetic surgical system, reducing the need for an additional assistant and enabling the retraction of large tissues and organs including the prostate and colon.
The company is aiming MARS at the high-volume abdominal surgery market. In Levita’s view, hospitals and ambulatory surgery centers that adopt the platform will benefit from increased efficiency and better deployment of operating room staff.