Dive Brief:
- Johnson & Johnson said Monday that a pivotal trial for the Impella ECP heart pump met its primary endpoint, positioning the company to seek Food and Drug Administration approval for the device.
- Impella ECP is designed to be easier to insert and implant than other heart pumps. The devices have become a key growth driver for J&J since it acquired Abiomed for $16.6 billion in late 2022.
- J&J reported a 6.3% rate of major adverse cardiac and cerebrovascular events after 30 days, which included death and stroke. The upper limit of the 95% confidence interval was below the predefined target, causing the trial to meet its primary endpoint.
Dive Insight:
Sales at J&J’s Abiomed business grew 16.3% in the third quarter. The company attributed the increase to growth across all regions and continued adoption of the surgically implanted Impella 5.5 and minimally invasive, right-sided heart pump Impella RP. The product line also features Impella CP, another minimally invasive pump, that provides support to patients undergoing high-risk percutaneous coronary intervention (PCI).
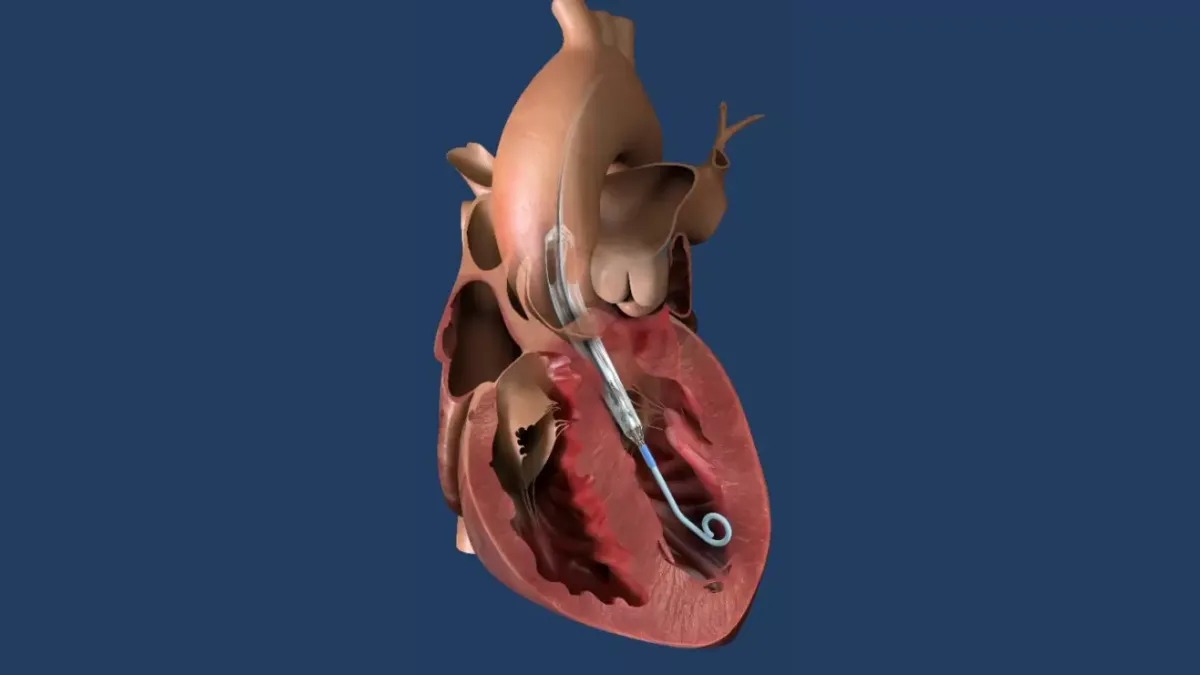
J&J is working to add Impella ECP to the portfolio. The device consists of an introducer sheath sized at 9-French in diameter, or 3 millimeters, and a pump sized at 21-French that is compressed for insertion and removal. Impella CP uses a 14-French sheath. J&J has predicted the narrower Impella ECP will be easier to insert and implant and enable the use of small bore access and closure techniques.
The company tested the device in a single-arm pivotal trial that enrolled 256 patients at 18 sites in the U.S. Patients were hemodynamically stable and eligible for high-risk PCI to open up blockages and restore blood flow to the heart.
After 30 days, the rate of major adverse cardiac and cerebrovascular events was 6.3%, below the 24.4% performance goal.
Amir Kaki, director of mechanical circulatory support and complex coronary intervention at Henry Ford-St. John Hospital and Medical Center, discussed the goal in his presentation of the data. The target was developed in conjunction with the FDA using prior data sets of high-risk PCI, said Kaki, who is the principal investigator of the study and a paid consultant for Abiomed.
J&J reported eight deaths, five strokes, four heart attacks and two target vessel revascularizations. The adverse events happened in 15 people, resulting in a rate of 6.3%. The upper limit of the 95% confidence interval, which needed to be below the performance goal for the trial to succeed, was 9.5%.
Nearly 6% of patients had major bleeding related to Impella ECP. The device was implicated in major vascular complications in 1.6% of patients. No patients had aortic valve injury or major hemolysis. Kaki highlighted the success of the wireless approach to delivering the device across the aortic valve.
“Despite our concerns about this novel approach to deliver the ECP across the aortic valve without a wire, it's remarkable to note that the average time required for the operators to cross the aortic valve was just over one minute, with a 100% success rate across multiple centers and multiple operators,” Kaki said.
The protocol allowed physicians to choose their approach to vascular closure. Most physicians used an 8-French Angio-Seal and that proved the most effective technique, achieving a success rate of 92%. The success rates for other techniques ranged from 72% to 80%.
Physicians have treated a further 243 patients through a continued access program since the trial ended. In total, J&J has data on 560 patients.