Dive Brief:
-
Johnson & Johnson has changed earlier plans to seek U.S. marketing authorization for its robotic platform for general surgery via the 510(k) pathway.
-
The decision, which J&J outlined on its second quarter earnings call Thursday, means the company is aiming to enter first-in-human clinical trials in the second half of 2022. J&J digital surgery venture Verb Surgical, which is contributing to the platform along with J&J-acquired Auris, at one point planned to introduce a product this year.
-
A planned regulatory submission for a separate robotics effort, its Velys orthopaedics platform, has also slipped slightly. But J&J remains upbeat about its prospects, noting that the market is still less than 5% penetrated.
Dive Insight:
J&J has invested in a number of digital surgery projects in recent years to position itself to claim a piece of a robotics market currently dominated by Intuitive Surgical.
In 2015, J&J set up a joint venture with Alphabet’s Verily Life Sciences, before going on to take full control of the business, Verb Surgical, at the end of last year. In between, J&J bought French surgical robot developer Orthotaxy for an undisclosed sum in 2018, and last year put up $3.4 billion to buy robotic endoscopy company Auris Health, created by the founder of Intuitive.
With J&J also investing in tumor-targeting platform Histosonics and forming a co-promotion pact with Chinese orrthopaedic robot maker Tinavi, the company has gained broad exposure to the robotic surgery market. The question now is whether J&J can generate a return on its investments.
In January, J&J said it plans to capture a piece of the general surgery market by combining elements of Auris and Verb’s platforms. That remains the plan, but J&J will have to wait to see whether it yields a commercially successful product.
“After analyzing time to market compared to overall value proposition, our goal is to initiate first-in-human studies with our robotic solution in the second half of 2022. The new timelines are based on what we know today, reflecting a world that is very different than what it was just a few short months ago,” CFO Joseph Wolk said on Thursday's call with investors.
J&J established the new timeline after its talks with regulators led it to opt against trying to bring its general surgery robotics platform to market via the 510(k) pathway. CEO Alex Gorsky sidestepped a request for further details on the investor call, choosing instead to reiterate what was said during the prepared remarks and talk up the size of the robotic surgery opportunity.
While acknowledging that robotic surgery is used in more than 50% of some procedures in the U.S., Gorsky said global penetration is below 5% and represents a “tremendous growth opportunity.” The general surgery platform is just one of the ways J&J is trying to seize that opportunity.
J&J also has the Velys digital surgery platform that it acquired in its takeover of Orthotaxy. Going into the year, J&J planned to file a U.S. regulatory submission for the orthopaedic robotics platform around the midpoint of the year. That target has now slipped slightly to the second half of 2020, with the initial focus being knee procedures.
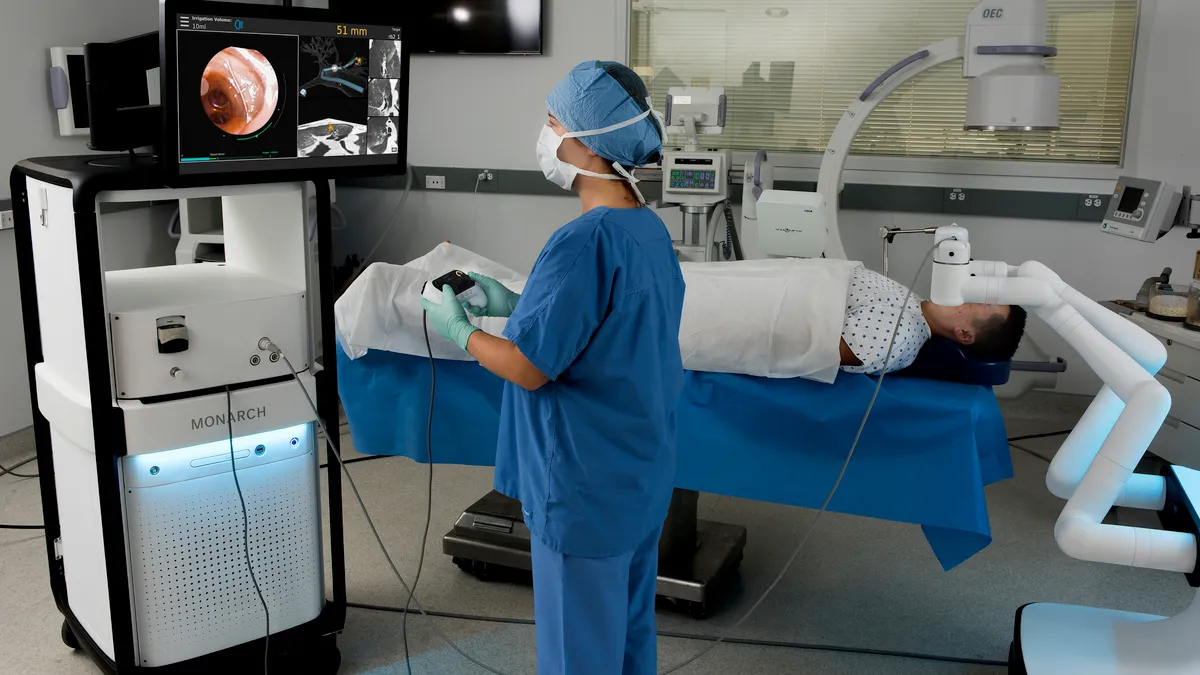
Monarch, the system J&J acquired from Auris, is already generating sales. J&J said surgeons have used the device to perform 3,200 lung biopsies. Now, J&J is working to get the device cleared for use in the treatment of kidney stones, a market that Wolk says is twice the size of its current addressable opportunity. The company also reported it received breakthrough device designation from FDA for its Monarch-enabled NeuWave ablation technology. J&J acquired minimally invasive soft tissue microwave ablation system maker NeuWave in 2016.
J&J entered into these robotic surgery markets around the same time as companies including Medtronic, Smith & Nephew, Stryker and Zimmer Biomet. These companies are all rolling out new products — Smith & Nephew disclosed a launch this week — and specialist rivals are raising money and bringing devices to market. Boston-based startup Activ Surgical raised $15 million this week to fund U.S. commercialization of its digital surgery platform.
The changes to robot timelines at J&J, in addition to a soft tissue robot delay at Medtronic, spell good news for Intuitive.
"This gives [Intuitive] more of a land grab [opportunity] ahead of competitive robot launches. Even though capital purchasing over next 6-12 months is likely to be limited, there is now extra time to go penetrate the market when capital purchasing resumes ahead of competitive launches likely in the 2022-2024 timeframe," analysts at SVB Leerink wrote in a research note Friday.