Dive Brief:
- Robotic surgery leader Intuitive Surgical said Friday its da Vinci single port system received FDA clearance for use in two otolaryngology procedures: radical tonsillectomy and tongue base resection.
- The transoral otolaryngology approval is the second procedure category to be cleared for the company's newcomer SP device. The da Vinci SP was initially cleared for urology procedures in May 2018.
- Separately, Intuitive appears to be making progress on increasing its international market penetration, according to research from UBS. The investment bank surveyed 105 hospital managers in Japan, Intuitive's second-largest market, and found that 10% were considering purchasing da Vinci systems.
Dive Insight:
Facing new competition on the horizon, Intuitive has answered by expanding its reach, pursuing growth both geographically and in new surgical specialties. On the back of an 18% increase in worldwide procedures using its systems in 2018, the company projects a slightly less torrid procedure growth pace of 13% to 17% in 2019.
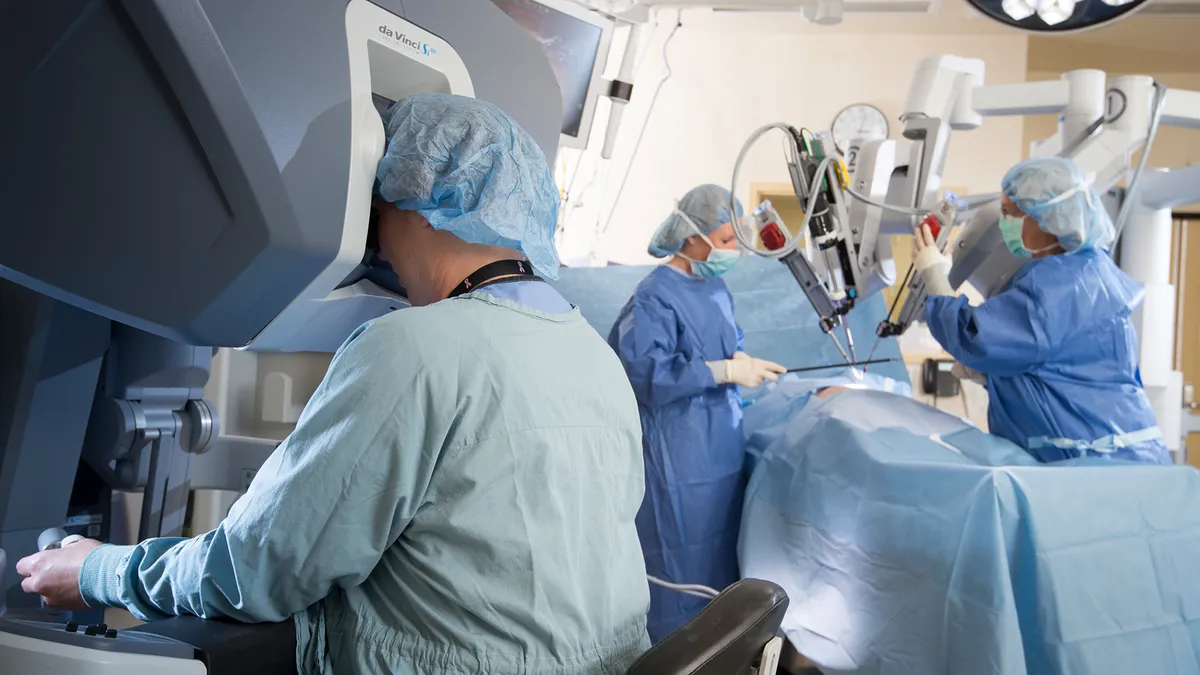
Unlike the original da Vinci robot that operates with four arms, the da Vinci SP's sole arm allows surgeons to guide the robot's instruments and camera through a single port in the patient's body. The SP is designed for deep and narrow access to tissue through a small incision or natural orifice.
The system has three multi-jointed instruments and a 3D HD camera that enter the body through a single cannula and are arranged around the target anatomy in a way that avoids instrument collisions that can occur in narrow surgical workspaces, the company said. Surgeons control the instruments and the camera on the da Vinci SP system using the same console as the da Vinci X and Xi systems.
With the additional indication, Intuitive said it will continue with its measured rollout of the SP system in 2019, following shipment of 15 of the devices in 2018.
Intuitive is also busy preparing for the introduction of its new Ion lung catheter that permits minimally invasive biopsies in the peripheral lung. The company has said it also plans a measured rollout of this device with customer shipments beginning in the second quarter, following the receipt of FDA clearance to market the product last month.
The company is stepping up efforts to sell more robotic systems overseas as well. On the company's fourth-quarter earnings call in January, CEO Gary Guthart said Intuitive planned to accelerate investment spending above its historical levels for the next several quarters to build its customer base in China, India and Taiwan in particular.
The company could be locking in on new business in Japan as well. According to the UBS report, six Japanese hospitals that already own da Vinci systems were considering buying additional robots, while four hospitals were thinking about buying a da Vinci for the first time. UBS said the Japanese government could grant premium reimbursement for gastric procedures using the robot in April 2020, providing a further catalyst for adoption of the system in the country.
Intuitive remains the dominant force in robotic surgery for abdominal procedures, but would-be competitors are coming on strong. Last month, Johnson & Johnson paid $3.4 billion in cash to acquire robotic technologies developer Auris Health. J&J's move follows Medtronic's purchase of Mazor Robotics for $1.7 billion in December. TransEnterix became the first abdominal robotics market competitor to Intuitive with FDA approval for its Senhance system in October 2017.